Figure 5.
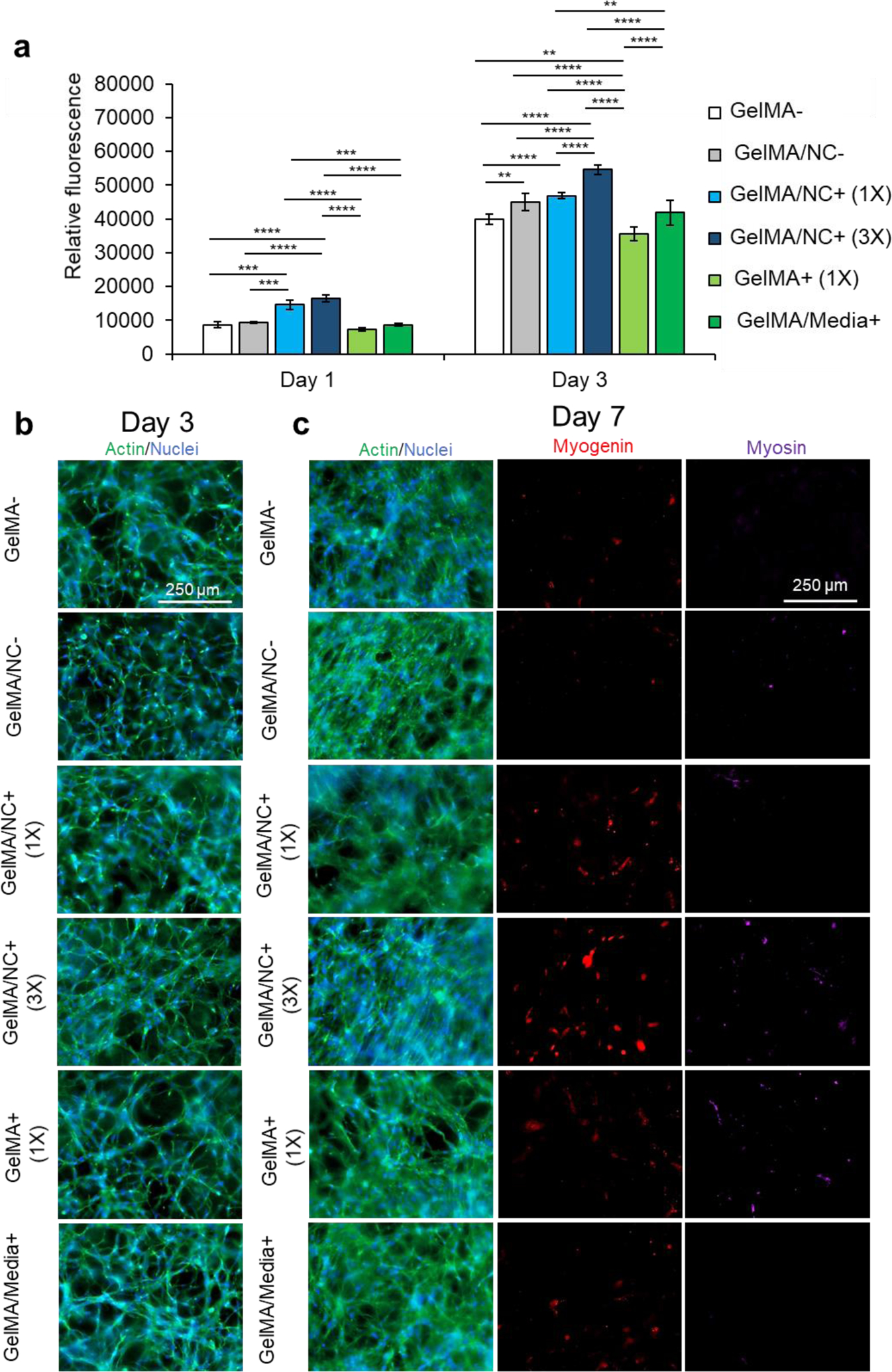
Proliferation studies within NC and control hydrogel conditions. (a) A PrestoBlue™ viability assay was used to determine the proliferative ability of C2C12 myoblasts encapsulated in varying GelMA-based hydrogel conditions: pristine GelMA (GelMA-), GelMA loaded with NC (GelMA/NC-), GelMA encapsulating 1X concentration of IGF-1 bound to NC (GelMA/NC+ (1X)), GelMA encapsulating 3X concentration of IGF-1 bound to NC (GelMA/NC+ (3X)), GelMA encapsulating IGF-1 (GelMA+ (1X)), and GelMA with IGF-1 added to the culture media (GelMA/Media+). On day 1, the GelMA/NC+ (1X) and GelMA/NC+ (3X) groups demonstrated the highest metabolic activity. On day 3 of culture, the GelMA/NC+ (3X) group had the most metabolic activity and was significantly different from all other groups. The lowest group was the GelMA+ (1X) group followed by the GelMA- control group. Exogenous IGF-1 added to the media in the GelMA/Media+ group did not appear to significantly increase the proliferation ability of the myoblasts relative to the GelMA- control. The GelMA/NC- control group demonstrated that the NC vehicle alone resulted in an increase in metabolic activity in association with previous studies. No significant difference was observed between the GelMA/NC- and the GelMA/1X groups on day 3. b) Actin (green) and nuclear (blue) staining of the hydrogel groups at 3 days of culture demonstrates the proliferative ability to vary IGF-1 delivery mechanisms. c) Actin (green) and nuclear (blue), myogenin (red), and myosin (purple) immunofluorescent images of myoblasts at day 7 of culture. Even without serum-deprived initiation of differentiation, the GelMA/NC+ (3X) condition initiated C2C12 differentiation displayed by the myogenin positive cells. Without induced differentiation, no apparent functional myotubes formed as evidenced by the negative myosin staining. Scale bars of 250 μm for all micrographs.
