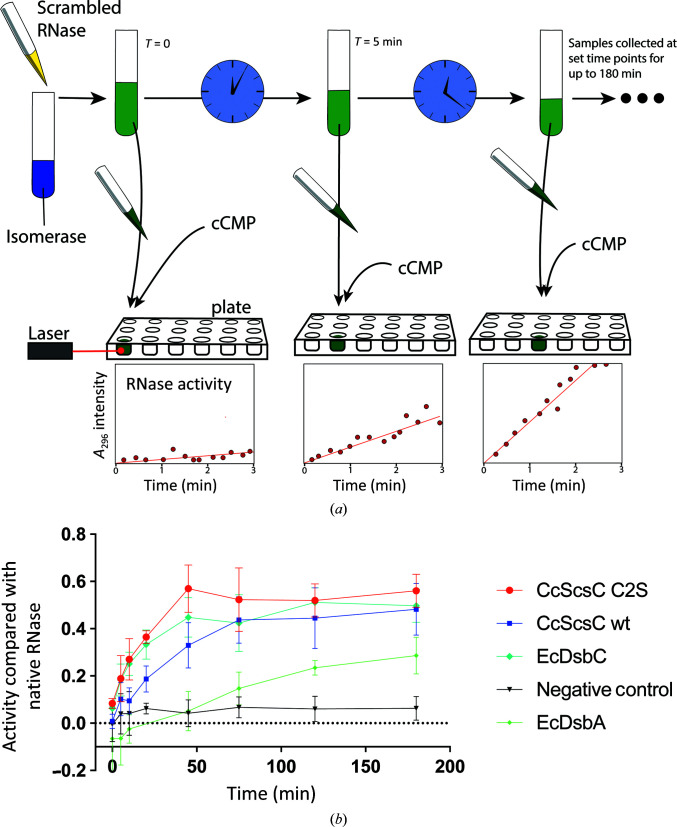
Figure 2.
Scrambled RNase (ScRNase) activity assay. (a) Schematic of the assay. ScRNase (yellow solution) containing eight cysteines forming four randomly formed disulfide bonds is mixed with dithiol oxidase or disulfide isomerase (blue solution). Over time, the isomerase (such as EcDsbC) will correct the scrambled disulfide bonds in RNase to form native (active) RNase. At specific time points, samples of the RNase/enzyme solution (green solution) are taken and mixed with cCMP in a microplate (samples collected at 0, 5, 10, 20, 45, 75, 90, 120 and 180 min). The RNase activity is measured by monitoring the absorbance at 296 nm over a 3 min time period, where the reaction rate is the fastest and data points are in the linear range. Active RNase hydrolyses cCMP, increasing the absorbance (at 296 nm) of the solution. The bottom plots are illustrative schematics of the increasing activity of RNase in hydrolysing cCMP over the duration of the experiment. (b) Absorbance measurements are presented as a ratio of the activity of each enzyme tested relative to the activity of native RNase. CcScsC wt and its variant CcScsC C2S show isomerase activities comparable to that of the positive control EcDsbC. Each measurement corresponds to the mean activity value (n = 5, except for EcDsbA, where n = 2; error bars correspond to the standard deviation of the mean). EcDsbA was used as an oxidase enzyme control as it is expected to have moderate activity in this assay. The negative control contained ScRNase without any enzyme.