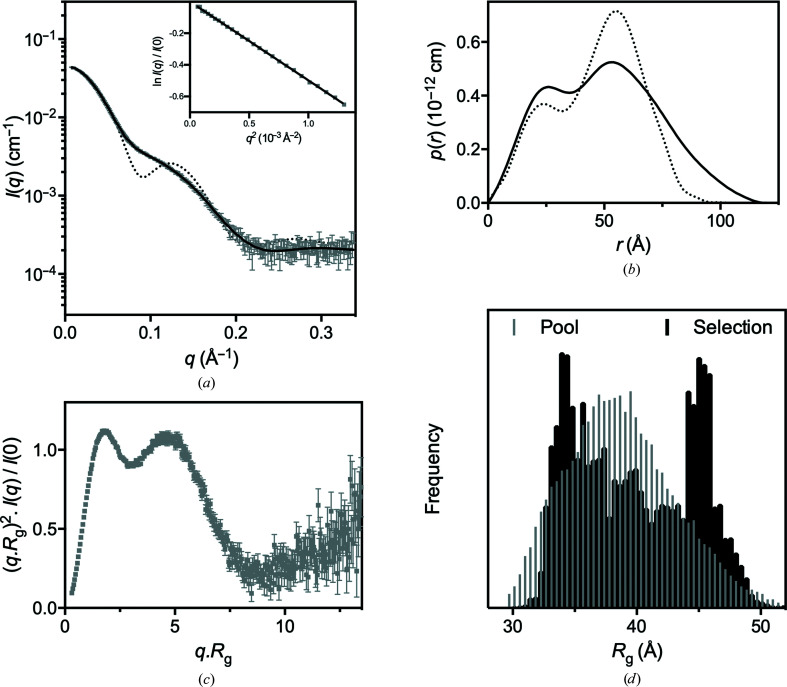
Figure 9.
Small-angle X-ray scattering data. (a) SAXS data collected from CcScsC wt (grey) with the calculated scattering profile of the ensemble model overlaid in black (SASBDB entry SASDLE9). The predicted scattering profile of the crystal structure is also shown (dotted line; PDB entry 7rgv). The agreement between the experimental data and the ensemble model is excellent, yielding χ2 = 1.32 (CorMap test P = 0.917) relative to the static rigid-body model (χ2 = 1.95; CorMap test P = 0.000) and the calculated scattering profile from the crystal structure (χ2 = 164; CorMap test P = 0.000). The Guinier region (inset) of the scattering data is linear, consistent with a monodisperse solution. (b) Pair-distance distribution function [p(r)] derived from the scattering data (solid line), showing that the maximum dimension of the particles in solution is 120 Å compared with 80 Å in the crystal trimer. Also shown is the calculated p(r) for the crystal structure (dotted line), which differs significantly from the experimentally determined SAXS solution p(r) curve. (c) The dimensionless Kratky plot calculated for the SAXS data. The first peak is consistent with a largely globular protein complex, while the second peak is due to the globular catalytic domains. At larger values of qR g the plot tends upwards, which is indicative of a flexible protein. (d) A histogram of the frequency as a function of the radius of gyration of the pool of structures (grey) and the selected structures (black). This plot shows that the ensemble analysis has preferentially selected structures with R g values of ∼35 and ∼45 Å relative to the pool population. This bimodal distribution is indicative of a molecule that is present mostly as a compact or extended molecule in solution.