ABSTRACT
Post-transplant diabetes mellitus (PTDM) is a common problem after kidney transplantation (KT), occurring in 50% of high-risk recipients. The clinical importance of PTDM lies in its impact as a significant risk factor for cardiovascular and chronic kidney disease (CKD) after solid organ transplantation. Kidney Disease: Improving Global Outcomes (KDIGO) has recently updated the treatment guidelines for diabetes management in CKD with emphasis on the newer antidiabetic agents such as dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonists and sodium–glucose co-transporter 2 inhibitors as add-on therapy to metformin. Given all these new diabetes treatments and the updated KDIGO guidelines, it is necessary to evaluate and give guidance on their use for DM management in KT recipients. This review summarizes the scarce published literature about the use of these new agents in the KT field. In summary, it is absolutely necessary to generate evidence in order to be able to safely use these new treatments in the KT population to improve blood glucose control, but specially to evaluate their potential cardiovascular and renal benefits that would seem to be independent of blood glucose control in PTDM patients.
Keywords: diabetes mellitus, dipeptidyl peptidase-4 inhibitors, incretins, kidney transplantation, sodium–glucose co-transporter 2 inhibitors
INTRODUCTION
Post-transplant diabetes mellitus (PTDM) is a common problem after kidney transplantation (KT) [1], occurring in 50% of high-risk recipients. The nomenclature of this disease changed after 2013; previously it was called new onset diabetes after transplantation (NODAT), but this term changed because it implied the exclusion of diabetes mellitus (DM) pre-transplantation, a problem that can potentially be unrecognized [2]. Currently, between preexisting DM and PTDM, approximately 50% of KT recipients require diabetic management [3].
Pre-transplant risk factors such as age, obesity, male gender, genetic background and hypertriglyceridaemia increase the risk of PTDM up to 50% [4]. In combination with some peritransplant triggers like immunosuppression, surgery stress, hypomagnesaemia and viral infections promote glucose intolerance and PTDM [5]. The pathophysiology of PTDM is related to β-cell damage; dysfunctional insulin release; impaired insulin-mediated glucose uptake in the peripheral tissue; impaired insulin-mediated suppression of hepatic glucose output secondary to disability of the incretin axis between the gut and pancreas; and impairment of brain regulation of the appetite, white fat mass and hepatic glucose output [1] (Figure 1).
FIGURE 1:
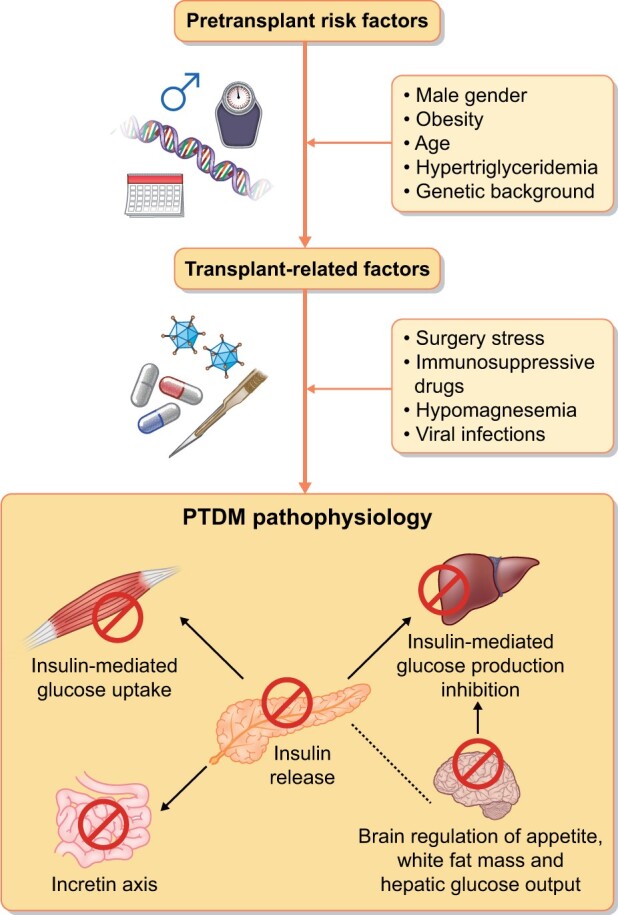
Pathophysiology of PTDM.
The clinical importance of PTDM lies in its unquestionable impact as a significant risk factor for cardiovascular disease (CVD) and chronic kidney disease (CKD) in solid organ transplantation [6]. Diabetic nephropathy is a major cause of CKD in liver transplants [7]. Also, diabetic nephropathy is found early after KT in patients with PTDM and is associated with kidney allograft failure [8]. The development of PTDM is a costly condition, with 67% increased risk of graft failure and an 87% increased risk of death due to premature CVD, cardiovascular deaths and infections [9]. Worse results are seen in those recipients with previous DM [10].
Kidney Disease: Improving Global Outcomes (KDIGO) has recently updated the treatment guidelines for diabetes management in CKD with emphasis on the newer antidiabetic agents such as dipeptidyl peptidase-4 inhibitors (DPP-4i), glucagon-like peptide-1 receptor agonists (GLP-1 RA) and sodium–glucose co-transporter 2 inhibitors (SGLT2i) as add-on therapy to metformin [11]. Some of these new agents show nephroprotective effects that are beyond glycaemic control.
In the early post-transplantation period, the most usual treatment is based on insulin analogues. This is usually challenging, since conditions change rapidly [changes in renal function, nutrition management, gastrointestinal (GI) motility disturbances or adjustment of immunosuppressive drugs]. In these recipients, it required intensive blood glucose monitoring and flexible and safe treatment algorithms. Oral drugs such as metformin are generally not used in transplant recipients due to their reduced renal function and the increased risk for metabolic acidosis.
Given all these interesting factors and the potential renoprotective effect of these agents, the appearance of new diabetes treatments and the updated KDIGO guidelines, it is necessary to evaluate and give guidance on DM management in KT recipients.
INCRETIN AGENTS
In this classification, we include GLP-1 RA and the DPP-4i. They stimulate β-cell function, slow gastric emptying and decrease insulin resistance [12]. GLP-1 RAs also suppress appetite. The use of them is associated with low risk of hypoglycaemia because of their glucose-dependent stimulation of insulin secretion [13].
Currently, there are six European Medicines Agency (EMA) approved GLP-1 RAs agents: exenatide, liraglutide, lixisenatide, albiglutide, semaglutide and dulaglutide, and five EMA-approved DPP-4i: sitagliptin, vildagliptin, saxagliptin, alogliptin and linagliptin [14].
Glucagon-like peptide-1 receptor agonists
There are two main molecules that control insulin secretion by β-cells following nutrient ingestion: glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 RA [15]. GLP-1 RA are structurally similar to endogenous GLP-1 released from gut enteroendocrine cells but have been engineered to be resistant to DPP-4 degradation [15]. GLP-1 RA exert their main effect by inhibiting inappropriate post-meal glucagon secretion and by suppressing hepatic glucose production, but they also delay gastric emptying and suppress central appetite. Compared with DPP-4i, haemoglobin A1c (HbA1c) reduction is higher, reported to be from 1% to 1.5% [16].
In case of type 2 DM, GLP-1 RA are preferred if there is a need to promote weight loss or also in patients with established CVD or heart failure [13].
Some selected published studies with GLP-1 RA use in KT are presented in Table 1.
Table 1.
Published studies with GLP-1 RA use in KT
| Study id | Study design, follow-up | Population | Intervention/s | Outcome |
|---|---|---|---|---|
| Pinelli et al. [17] |
|
KT recipients with or without previous DM or PTDM, with stable renal function receiving tacrolimus | All patients received liraglutide in monotherapy |
|
| Halden et al. [18] |
|
KT with and without PTDM | Intravenous infusion of GLP-1 versus saline (placebo) |
|
| Liou et al. [19] |
|
KT recipients with PTDM treated with liraglutide | All patients received liraglutide |
|
| Singh et al. [20] |
|
|
All patients received dulaglutide |
|
| Singh et al. [21] |
|
|
All patients received dulaglutide or liraglutide |
|
FBS, fasting blood sugar; SOT, solid organ transplant.
In case of PTDM, GLP-1 RAs have the following potential benefits:
-
Metabolic impact: increases insulin secretion and reduces glucagon secretion. This effect has been shown in the Halden et al. [18] study, which compared 12 KT recipients with PTDM and 12 without. Infusions of GLP-1 were compared with saline infusions, establishing a hyperglycaemic clamp. The authors characterized PTDM by a reduced glucose-induced insulin secretion and attenuated glucagon suppression with improvement of both defects by GLP-1 infusion (reduction in glucagon levels in GLP-1 group: −22 ± 15%; P = 0.007, and improved maximal insulin stimulation by 102%; P = 0.003). Published studies in PTDM recipients show a similar effect on glucose control using GLP1-AR compared with the general type 2 DMpopulation. For example, in a brief case series including seven KT recipients with PTDM, the addition of liraglutide to other antidiabetic agents entailed a significant reduction of HbA1c from 10.04% to 8.14% (P = 0.047) [19]. This beneficial effect on glucose control is similar between the different molecules, but reductions on daily insulin requirements are more pronounced using dulaglutide compared with liraglutide (−26% versus −3.6%; P = 0.01) in the solid organ transplant population, which may be related to dulaglutide’s longer duration of action [21].
In the general type 2 DM population, there are differences in efficacy in terms of weight loss depending on the GLP-1 RA agent. Although there are few randomized controlled trials (RCTs) that directly compare the relative efficacy of drugs in weight loss, the available head-to-head comparisons (semaglutide versus exenatide extended release/dulaglutide/liraglutide; exenatide immediate release versus dulaglutide and dulaglutide versus exenatide/albiglutide), it has been shown that semaglutide is the most potent molecule in terms of weight reduction in comparison with the rest of the GLP-1 RA [22, 23]. Results in studies including KT recipients only evaluated differences between dulaglutide versus liraglutide and they also confirmed this reduction of weight at 24 months with liraglutide use [21].
Cardiovascular effects: reducing cardiovascular events. A benefit in terms of reducing cardiovascular events has been shown in most of the published studies in the type 2 DM population [24–27]. In the KT population, only two retrospective studies reported the number of cardiovascular events, but without being able to reach any conclusion [20, 21].
Kidney effects: increase of renoprotection. These effects have been largely demonstrated in type 2 DM trials [24, 26]. In this population, in terms of kidney function improvements, the use of GLP-1 RAs reduced the urine albumin-to-creatinine ratio and slowed estimated glomerular filtration rate (eGFR) decline [24, 25, 28, 29]. In KT, results have not been so overwhelming and with differences depending on the GLP-1 RA agent. In a retrospective study including 63 patients, the use of dulaglutide resulted in no significant changes in eGFR after 24 months (in 13 patients: +6.54 mL/min/1.73 m2; P = 0.07) [20]. These results were not confirmed in another cohort of 25 PTDM recipients treated with liraglutide, the authors justified this discrepancy because of a potential increase of glomerular hyperfiltration in obese recipients [21].
Adverse effects related to GLP-1 RA use are headaches, injection site pain and, importantly in KT recipients, mild GI intolerances (nausea and reduced appetite) that can potentially be worsened by concomitant use of mycophenolate [19]. Because these GI side effects are mediated by different mechanisms—in GLP-1 RA by suppression of central appetite and delay of gastric emptying and in mycophenolate by the inhibition of the replication of GI epithelial cells, leading to disruption of fluid absorption and diarrhoea—they can be potentiated by the concurrent use of the two types of drugs.
There are no interactions with immunosuppressants mediated by CYP (cytochrome P450 enzymes) or transporters [30] (Supplementary data, Table S1) [31–33]. Some observational studies showed that delays in gastric emptying related to GLP-1 RAs did not alter levels or doses of immunosuppression [19].
Each GLP-1 RA agent has different posology: exenatide twice a day (should be administered within 60 min before main meals); liraglutide once daily (QD) (at any time, without regard to meals); lixisenatide QD (should be administered within 60 min before main meals); or exenatide, albiglutide, semaglutide and dulaglutide each given once weekly [14]. GLP-1 RAs are cleared by proteolytic degradation and glomerular filtration, so dose adjustments depending on kidney function are required with exenatide and lixisenatide.
Dipeptidyl peptidase-4 inhibitors
DPP-4i act indirectly by blocking proteolytic cleavage of GLP-1 by DPP-4 [34]. This generates a glucose-lowering effect, but apart from that, it also promotes β-cell proliferation, neogenesis and inhibition of apoptosis [35].
Some selected published studies with DPP-4i use in KT are presented in Table 2.
Table 2.
Published studies with DPP-4i use in KT
| Study id | Study design, follow-up | Population | Intervention/s | Outcome |
|---|---|---|---|---|
| Lane et al. [36] |
|
KT recipients with eGFR >30 mL/min/1.73 m2 and diagnosis of PTDM | All patients treated with sitagliptin |
|
| Sanyal et al. [37] |
|
|
All patients received linagliptin monotherapy (5 mg/day) |
|
| Soliman et al. [38] |
|
KT recipients with PTDM receiving metformin and inadequate glycaemic control |
|
|
| Boerner et al. [39] |
|
KT recipients with diagnosis of PTDM treated with sitagliptin alone | All patients treated with sitagliptin monotherapy |
|
| Haidinger et al. [40] |
|
KT recipients (>6 months post-KT) with stable renal function and diagnosis of PTDM | Vildagliptin 50 mg/day versus placebo during 3 months |
|
| Strøm Halden et al. [41] |
|
KT recipients (>1a) with PTDM and stable renal function |
|
|
| Guardado-Mendoza et al. [42] |
|
KT recipients with fasting hyperglicaemia during the first 24 h post-surgery | Linagliptin 5 mg/days plus insulin versus insulin alone |
|
FPG, fasting plasma glucose.
In the case of PTDM, DPP-4i have these potential roles:
-
Metabolic impact:
Repairing pathophysiological aetiologies of insulin resistance and β-cell dysfunction: there are two published studies that show an improvement of insulin resistance with DPP-4i treatment in the early KT. Thiruvengadam et al. [34] compared the use of linagliptin (n = 19) versus other therapies (metformin, insulin or sulphonylureas) (n = 21) after the early diagnosis of PTDM, showing an improvement of insulin resistance [evaluated by the calculated homeostatic model assessment for insulin resistance (HOMA-IR) scores (2.21 versus 3.33; P = 0.02)]. This effect was also shown in the Strøm Halden study [41], including 19 patients who were randomly assigned to be treated for 4 weeks with sitagliptin just after the diagnosis of diabetes followed by 4 weeks of no treatment or vice versa. The authors described an increased insulin secretion response and also an increased insulin sensitivity with sitagliptin (median sensitivity increase of 25.3%; P = 0.04).
Use as adjunctive therapy: lowering insulin requirements early post-transplantation. One of the main pathologic pathways of PTDM appearance is because of impaired insulin secretion. As opposed to sulphonylureas, DPP-4i improve insulin response without aggravating β-cell decline via islet cell exhaustion. This has been shown in some cohort studies by a significant increase in C-peptide values corrected for creatinine and glucose indicating that DPP-4i improved β-cell function in KT recipients [41] but also in type 2 DM patients. In another study, the authors showed lower requirements of insulin doses with combined linagliptin use than insulin alone (24.2 versus 37.5 daily units of insulin; P < 0.05) [42]. All these mechanisms lead to a better control of PTDM, achieving a maintained reduction of HbA1c [36, 39, 40] and an improvement of 2-h plasma glucose (2HPG) on oral glucose tolerance test [37, 40].
Reducing obesity: obesity is related to worse graft and patient outcomes in the short and long term after transplantation [43]. One of the potential effects of long-term treatment with DPP-4i is body mass index reduction, which has been shown in one case series study evaluating the use of sitagliptin in a cohort of 22 recipients, after 12 months of treatment (−0.8 kg/m2; P < 0.05) [39].
Cardiovascular effects: in the general population, studies using these agents did not show any impact on major adverse cardiovascular events including myocardial infarction, stroke and cardiovascular death [44–47]. In KT, there are no available studies evaluating this outcome.
Kidney effects: in the general type 2 diabetic population, there have been shown a reduction of albuminuria with the use of saxagliptin [44] and linagliptin [48] without data reported about effects on renal function. In KT population, no information has been reported of the effects of these molecules on albuminuria, but four articles reported no differences in kidney function [36, 37, 39, 44].
Few adverse effects have been described with the use of DPP-4i, with GI intolerances rarely reported.
There are five EMA-approved DPP-4i (sitagliptin, vildagliptin, saxagliptin, alogliptin and linagliptin), with individual pharmacokinetics (PK) characteristics. There are few known drug interactions with immunosuppressants. However, in the case of KT, initiation of these compounds may require closer monitoring of immunosuppression levels [49]. Depending on the molecule, they can be a P-glycoprotein (PGP) substrate and/or CYP3A4/5 substrate, increasing the likelihood for drug interactions with immunosuppressants [49]. Currently, there are no studies designed specifically to evaluate this potential interaction (Supplementary data, Table S2) [49, 50]. The PK differ depending on the drug concerned: half-life, binding to plasma proteins, the presence of active versus inactive metabolites, predominant renal versus hepatic excretion (necessity of dosage adjustment in case of renal or liver impairment) and propensity for drug–drug interactions [51]. The PK of DPP-4i have been studied in healthy young male subjects, patients with type 2 DM and patients with either renal insufficiency or hepatic impairment [51]. Doses adjustments depending on eGFR are required for sitagliptin, saxagliptin and alogliptin with a reduction of the dose from eGFR <45 mL/min/1.73 m2.
SODIUM–GLUCOSE CO-TRANSPORTER 2 INHIBITORS
The benefits related to SGLT2i use in terms of kidney function have been widely reported in type 2 DM. Five relevant clinical trials in general type 2 diabetic population (EMPA-REG [52], CANVAS [53], DECLARE [54, 55], CREDENCE [56] and DAPA-CKD [57]) have shown that treatment with SGLT2i is capable of slowing the progression of CKD and the appearance of renal events. The mechanism of action is by increasing urine glucose excretion [58] by inhibiting a low-affinity transport system called SGLT2 that leads renal glucose reabsorption in the proximal tubule of the kidneys. This inhibition of glucose absorption has two benefits: it improves glycaemia control and it also reduces obesity by enhancing glucose and energy loss through the urine by a non-insulin-dependent mechanism of action and these effects improve kidney and cardiovascular outcomes.
Some selected published studies with SGLT2i use in KT are presented in Table 3.
Table 3.
Published studies with SGLT2i use in KT
| Study id | Study design, follow-up | Population | Intervention/s | Outcome |
|---|---|---|---|---|
| Rajasekeran et al. [59] |
|
KT (n = 6) and SPKT (n = 4) recipients treated with canaglifozin | All patients treated with canaglifozin |
|
| Schwaiger et al. [60] |
|
KT with PTDM receiving treatment with insulin and eGFR >30 mL/min/1.73 m2 |
|
|
| Attallah et al. [61] |
|
KT treated with empaglifozin (previous DM n = 4, PTDM n = 4) |
|
|
| Halden et al. [62] |
|
KT recipients with diagnosis of PTDM | Empaglifozin (n = 22) versus placebo (n = 22) |
|
| Mahling et al. [63] |
|
|
All patients received empaglifozin |
|
FPG, fasting plasma glucose; IQR, interquartile range; SPKT, simultaneous pancreas-kidney transplant.
In case of PTDM, SGLT2i have these potential roles:
Metabolic impact: reducing obesity. Based on small trials, the effect of SGLT2i on lowering HbA1c in KT recipients is modest and it depends on kidney function, but the beneficial effect on renal function appears to be independent of glycaemic control and could be seen in eGFR ≥20 mL/min/1.73 m2. The improvement of metabolic control of PTDM is seen when SLGT2i is added to other antidiabetic medications. In the KT population using empagliflozin compared with placebo, a significant weight loss of −1.6 kg after 4 weeks (P = 0.02), −5 kg at 12 weeks [60] and −2.5 kg (P = 0.014) after 12 months [62] has been shown.
Cardiovascular effects: improving cardiovascular outcomes after KT. There are two studies in type 2 diabetic population that proved their ability to lower systolic blood pressure and reduce cardiovascular deaths and heart failure hospitalizations [52, 54]. In a cohort of 7020 patients, the EMPA-REG RCT [52], having poor control of blood pressure levels (>160/100 mmHg) was an exclusion criteria. Most of the included population used renin–angiotensin–aldosterone system (RAAS) blockers before starting the trial and, without specifying if changes of antihypertensive treatment were done, a blood pressure reduction of −3 mmHg was shown with SGLT2i use at the end of the study. In the other RCT including 17 160 patients, the DECLARE-TIMI 58 [54, 55], the authors described similar levels of basal blood pressure with the same percentage of RAAS blockers, beta-blocker or diuretics use, and a significant decrease of systolic blood pressure of 2.7 mmHg and diastolic blood pressure of 0.7 mmHg was seen in the SGLT2i group. In the KT population, this effect of lowering blood pressure was showed only in one observational case-series study with 10 KT recipients treated with empagliflozin, which found a significant reduction of −8 mmHg in systolic blood pressure (P < 0.05), but also the number of antihypertensive was increased from 3 to 4 [60], whereas two other case series of 10 KT each one using canagliflozin [59] and empagliflozin [63], where the authors specified that antihypertensive therapy could be modified by the treating physician if necessary, they found no effect. An RCT including 40 KT recipients randomized to receive empagliflozin or placebo, with no differences in basal blood pressure levels or number of antihypertensive therapies, 24-h blood pressure measurements revealed no significant differences in systolic or diastolic blood pressure, or pulse [62]. These different effects compared with the non-KT diabetic population may be explained by different pathogenic mechanisms of CVD that depend not only on diabetes, but also on adverse effects directly related to immunosuppression.
-
Kidney effects: improving renal function and outcomes of the graft. Renoprotective effects are related to SGLT2i-induced natriuresis by reducing proximal tubular sodium reabsorption and consequently increasing distal sodium delivery to the macula densa, activating tubulo-glomerular feedback, increasing afferent arteriolar tone, reducing renal perfusion, lowering glomerular pressure and reducing hyperfiltration [64]. The same mechanism may be present in KT recipients, but to our knowledge it is unknown and confirming studies are needed.
An increase of serum creatinine (SCr) and a decrease of eGFR are seen with the initiation of SGLT2i also in recipients with PTDM. This effect has been reported in two studies using empagliflozin: in a study including 14 recipients treated with this agent a reduction of eGFR from 55.6 to 47.5 mL/min/1.73 m2 was shown at 4 weeks [60] and in another contemporary eight case-series report, similar increases in SCr were seen after 4 weeks (from 88.5 to 99.5 mmol/L). A stabilization of eGFR after this initial increase at 12 months has been confirmed in three studies [59, 61, 63]. A reduction of proteinuria has been reported only in one study, with a mean decrease of 0.6 g/day after 1 year [61].
In terms of adverse effects [14] data based on studies of general type 2 DM, the most frequent one is urinary tract infection (UTI). This is especially relevant in the KT population, who are more vulnerable because of chronic immunosuppression and genitourinary structural or functional abnormalities after the surgery. In this population, UTI can lead to a deterioration of renal function and, in the worst cases, graft loss. It has also been described that SGLT2i use in general DM recipients can be associated with genital mycotic infections and necrotizing fasciitis, which can be particularly harmful in immunosuppressed KT recipients. The risk of lower-limb amputation has been refuted in more recent studies [52–54, 56]. In studies focused on a few number of KT recipients, there were no more UTIs with SGLT2i use (incidences between 20% and 25%) [60, 61, 63], but due to the low number of patients included in these studies, these results have to be read with caution. Due to the absence of information in this KT population, it seems reasonable to avoid the use of these treatments in KT recipients with recurrent UTIs.
There are four EMA-approved DPP-4i (canagliflozin, dapagliflozin, empagliflozin and ertugliflozin). There are few known drug interactions with immunosuppressants and SGLT2i, with the exception of canagliflozin (Supplementary data, Table S3) [65, 66]. All SGLT2i are PGP substrates, but the only documented interaction is with canagliflozin use is a weak PGP inhibitor, which could result in increased calcineurin inhibitor and mycophenolate levels [30]. It is important to highlight that the only SGLT2i that can be given with eGFR ≤45 mL/min/1.73 m2 is canagliflozin, with a dose of 100 mg orally daily (Supplementary data, Table S3) [14].
CONCLUSIONS
Seven recommendations for PTDM were proposed in the last meeting report of the American Diabetes Association, one of which was: ‘to adopt strategies for prevention and treatment beyond modification of immunosuppressive regimen’ [2]. To date, the only published study that evaluates a strategy to prevent PTDM showed that the use of insulin to treat hyperglycaemia in the immediate post-transplant period reduced the odds of persistent PTDM in the first year post-KT by 73% but with a higher number of hypoglycaemic episodes [67]. The treatment with insulin analogs in the early post-transplantation period is challenging since clinical conditions are changing rapidly (changes in renal function, nutrition management, GI motility disturbances or adjustment of immunosuppressive drugs). Thus, in these recipients, an intensive blood glucose monitoring is required with flexible and safe treatment algorithms.
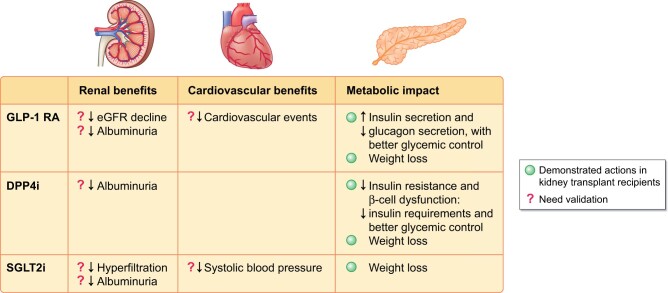
Apart from the referred scarce published literature about the use of incretin agents and SGLT2i in PTDM, there are four ongoing studies that are presented in Supplementary data, Table S4. The studies showed their potential beneficial effect for the KT population in terms of control of glucose and cardiovascular risk factors. Nevertheless, whether the use of incretin agents and SGTL2i is associated with nephroprotection and with any effect on cardiovascular outcomes in solid organ transplant deserves further attention. Efficacy beyond glucose has not been conclusively demonstrated in the KT population (Figure 2). Moreover, it is necessary also to focus on evaluating particularly PK outcomes (Supplementary data, Table S5) and interaction with immunosuppression.
FIGURE 2:
Summary of renal, cardiovascular and metabolic actions of GLP-1 RA, DPP-4i and SLGLT2i.
In summary, it is absolutely necessary to generate evidence in order to be able to safely use these new treatments in KT population and to establish a broader therapeutic strategy to improve blood glucose control and evaluate the role of these new agents in terms of cardiovascular and renal benefits, that would seem to be independent of blood glucose control, and in terms of their potential role as preventive strategies to avoid the appearance of PTDM. Subsequently, research needs might be focused on evaluating the effect of these agents on (i) kidney outcomes, (ii) cardiovascular outcomes and (iii) immunosuppression interaction, which could be addressed by RCTs comparing each of these molecules with the actual standard of care. Based on the effectiveness shown in the general type 2 DM population and the great incidence of cardiovascular outcomes in KT population, the sample size required would be feasible if a multicentric study is planned. Therefore, a call for clinical trials on PTDM treatment seems urgently needed.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
CONFLICT OF INTEREST STATEMENT
Maria José Soler and Josep Maria Cruzado are Members of the CKJ Editorial Board.
Supplementary Material
Contributor Information
Nuria Montero, Department of Nephrology, L’Hospitalet de Llobregat, Hospital Universitari de Bellvitge, Barcelona, Spain; Biomedical Research Institute (IDIBELL), L’Hospitalet de Llobregat, University of Barcelona, Barcelona, Spain.
Laia Oliveras, Department of Nephrology, L’Hospitalet de Llobregat, Hospital Universitari de Bellvitge, Barcelona, Spain.
Maria José Soler, Department of Nephrology, Hospital Vall d’Hebron, Barcelona, Spain.
Josep Maria Cruzado, Department of Nephrology, L’Hospitalet de Llobregat, Hospital Universitari de Bellvitge, Barcelona, Spain; Biomedical Research Institute (IDIBELL), L’Hospitalet de Llobregat, University of Barcelona, Barcelona, Spain.
REFERENCES
- 1. Jenssen T, Hartmann A. Post-transplant diabetes mellitus in patients with solid organ transplants. Nat Rev Endocrinol 2019; 15: 172–188 [DOI] [PubMed] [Google Scholar]
- 2. Sharif A, Hecking M, de Vries APJ et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: Recommendations and future directions. Am J Transplant 2014; 14: 1992–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hart A, Smith JM, Skeans MA et al. OPTN/SRTR 2018 annual data report: Kidney. Am J Transplant 2020; 20: 20–130 [DOI] [PubMed] [Google Scholar]
- 4. Chakkera HA, Chang Y-H, Ayub A et al. Validation of a pretransplant risk score for new-onset diabetes after kidney transplantation. Diabetes Care 2013; 36: 2881–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Montero N, Pascual J. Immunosuppression and post-transplant hyperglycemia. Curr Diab Rev 2015; 11: 144–154 [DOI] [PubMed] [Google Scholar]
- 6. Hjelmesæth J, Hartmann A, Leivestad T et al. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int 2006; 69: 588–595 [DOI] [PubMed] [Google Scholar]
- 7. Longenecker JC, Estrella MM, Segev DL et al. Patterns of kidney function before and after orthotopic liver transplant: Associations with length of hospital stay, progression to end-stage renal disease, and mortality. Transplantation 2015; 99: 2556–2564 [DOI] [PubMed] [Google Scholar]
- 8. Nagib AM, Elsayed Matter Y, Gheith OA et al. Diabetic nephropathy following posttransplant diabetes mellitus. Exp Clin Transplant 2019; 17: 138–146 [DOI] [PubMed] [Google Scholar]
- 9. Hecking M, Sharif A, Eller K et al. Management of post-transplant diabetes: Immunosuppression, early prevention, and novel antidiabetics. Transpl Int 2021; 34: 27–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuo H-T, Sampaio MS, Vincenti F et al. Associations of pretransplant diabetes mellitus, new-onset diabetes after transplant, and acute rejection with transplant outcomes: An analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing (OPTN/UNOS) database. Am J Kidney Dis 2010; 56: 1127–1139 [DOI] [PubMed] [Google Scholar]
- 11. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020; 98: S1–S115 (doi: 10.1016/j.kint.2020.06.019) [DOI] [PubMed] [Google Scholar]
- 12. Drucker DJ. The biology of incretin hormones. Cell Metab 2006; 3: 153–165 [DOI] [PubMed] [Google Scholar]
- 13. MacDonald PE, El-Kholy W, Riedel MJ et al. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 2002; 51 (Suppl 3): S434–S442 [DOI] [PubMed] [Google Scholar]
- 14. European Medicines Agency. https://www.ema.europa.eu/en (31 March 2021, date last accessed)
- 15. Sadhu AR, Schwartz SS, Herman ME. The rationale for use of incretins in the management of new onset diabetes after transplantation (NODAT). Endocr Pract 2015; 21: 814–822 [DOI] [PubMed] [Google Scholar]
- 16. Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: A review of their efficacy and tolerability. Diabetes Care 2011; 34 (Suppl 2): S279–S284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pinelli NR, Patel A, Salinitri FD. Coadministration of liraglutide with tacrolimus in kidney transplant recipients: A case series. Diabetes Care 2013; 36: e171–e172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Halden TAS, Egeland EJ, Åsberg A et al. GLP-1 restores altered insulin and glucagon secretion in posttransplantation diabetes. Diabetes Care 2016; 39: 617–624 [DOI] [PubMed] [Google Scholar]
- 19. Liou J-H, Liu Y-M, Chen C-H. Management of diabetes mellitus with glucagonlike peptide-1 agonist liraglutide in renal transplant recipients: A retrospective study. Transplant Proc 2018; 50: 2502–2505 [DOI] [PubMed] [Google Scholar]
- 20. Singh P, Pesavento TE, Washburn K et al. Largest single-centre experience of dulaglutide for management of diabetes mellitus in solid organ transplant recipients. Diabetes Obes Metab 2019; 21: 1061–1065 [DOI] [PubMed] [Google Scholar]
- 21. Singh P, Taufeeq M, Pesavento TE et al. Comparison of the glucagon-like-peptide-1 receptor agonists dulaglutide and liraglutide for the management of diabetes in solid organ transplant: A retrospective study. Diabetes Obes Metab 2020; 22: 879–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Capehorn MS, Catarig A-M, Furberg JK et al. Efficacy and safety of once-weekly semaglutide 1.0 mg vs once-daily liraglutide 1.2 mg as add-on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab 2020; 46: 100–109 [DOI] [PubMed] [Google Scholar]
- 23. Lyseng-Williamson KA. Glucagon-like peptide-1 receptor analogues in type 2 diabetes: Their use and differential features. Clin Drug Investig 2019; 39: 805–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mann JFE, Ørsted DD, Brown-Frandsen K et al. ; LEADER Steering Committee and Investigators. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 2017; 377: 839–848 [DOI] [PubMed] [Google Scholar]
- 25. Marso SP, Bain SC, Consoli A et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–1844 [DOI] [PubMed] [Google Scholar]
- 26. Gerstein HC, Colhoun HM, Dagenais GR et al. Dulaglutide and renal outcomes in type 2 diabetes: An exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 2019; 394: 131–138 [DOI] [PubMed] [Google Scholar]
- 27. Hernandez AF, Green JB, Janmohamed S et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. Lancet 2018; 392: 1519–1529 [DOI] [PubMed] [Google Scholar]
- 28. Muskiet MHA, Tonneijck L, Huang Y et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: An exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2018; 6: 859–869 [DOI] [PubMed] [Google Scholar]
- 29. Tuttle KR, Lakshmanan MC, Rayner B et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): A multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol 2018; 6: 605–617 [DOI] [PubMed] [Google Scholar]
- 30. Anderson S, Cotiguala L, Tischer S et al. Review of newer antidiabetic agents for diabetes management in kidney transplant recipients. Ann Pharmacother 2020; 55: 496–508 [DOI] [PubMed] [Google Scholar]
- 31. Muskiet MHA, Tonneijck L, Smits MM et al. GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol 2017; 13: 605–628 [DOI] [PubMed] [Google Scholar]
- 32. van Baar MJB, van der Aart AB, Hoogenberg K et al. The incretin pathway as a therapeutic target in diabetic kidney disease: A clinical focus on GLP-1 receptor agonists. Ther Adv Endocrinol Metab 2019; 10: 2042018819865398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scheen AJ. Cardiovascular effects of new oral glucose-lowering agents: DPP-4 and SGLT-2 inhibitors. Circ Res 2018; 122: 1439–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thiruvengadam S, Hutchison B, Lim W et al. Intensive monitoring for post-transplant diabetes mellitus and treatment with dipeptidyl peptidase-4 inhibitor therapy. Diabetes Metab Syndr 2019; 13: 1857–1863 [DOI] [PubMed] [Google Scholar]
- 35. Marrano N, Biondi G, Cignarelli A et al. Functional loss of pancreatic islets in type 2 diabetes: How can we halt it? Metabolism 2020; 110: 154304. [DOI] [PubMed] [Google Scholar]
- 36. Lane JT, Odegaard DE, Haire CE et al. Sitagliptin therapy in kidney transplant recipients with new-onset diabetes after transplantation. Transplantation 2011; 92: e56–e57 [DOI] [PubMed] [Google Scholar]
- 37. Sanyal D, Gupta S, Das P. A retrospective study evaluating efficacy and safety of linagliptin in treatment of NODAT (in renal transplant recipients) in a real world setting. Indian J Endocrinol Metab 2013; 17: S203–S205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Soliman AR, Fathy A, Khashab S et al. Sitagliptin might be a favorable antiobesity drug for new onset diabetes after a renal transplant. Exp Clin Transplant 2013; 11: 494–498 [DOI] [PubMed] [Google Scholar]
- 39. Boerner BP, Miles CD, Shivaswamy V. Efficacy and safety of sitagliptin for the treatment of new-onset diabetes after renal transplantation. Int J Endocrinol 2014; 2014: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haidinger M, Werzowa J, Hecking M et al. Efficacy and safety of vildagliptin in new-onset diabetes after kidney transplantation—a randomized, double-blind, placebo-controlled trial. Am J Transplant 2014; 14: 115–123 [DOI] [PubMed] [Google Scholar]
- 41. Strøm Halden TA, Åsberg A, Vik K et al. Short-term efficacy and safety of sitagliptin treatment in long-term stable renal recipients with new-onset diabetes after transplantation. Nephrol Dial Transplant 2014; 29: 926–933 [DOI] [PubMed] [Google Scholar]
- 42. Guardado-Mendoza R, Cázares-Sánchez D, Evia-Viscarra ML et al. Linagliptin plus insulin for hyperglycemia immediately after renal transplantation: A comparative study. Diabetes Res Clin Pract 2019; 156: 107864. [DOI] [PubMed] [Google Scholar]
- 43. Montero N, Quero M, Arcos E et al. Effects of body weight variation in obese kidney recipients: A retrospective cohort study. Clin Kidney J 2020; 13: 1068–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scirica BM, Bhatt DL, Braunwald E et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369: 1317–1326 [DOI] [PubMed] [Google Scholar]
- 45. Zannad F, Cannon CP, Cushman WC et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: A multicentre, randomised, double-blind trial. Lancet 2015; 385: 2067–2076 [DOI] [PubMed] [Google Scholar]
- 46. Green JB, Bethel MA, Armstrong PW et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 373: 232–242 [DOI] [PubMed] [Google Scholar]
- 47. Rosenstock J, Perkovic V, Johansen OE et al. ; for the CARMELINA Investigators. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: The CARMELINA randomized clinical trial. JAMA 2019; 321: 69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Perkovic V, Toto R, Cooper ME et al. Effects of linagliptin on cardiovascular and kidney outcomes in people with normal and reduced kidney function: Secondary analysis of the CARMELINA randomized trial. Diabetes Care 2020; 43: 1803–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Scheen AJ. Dipeptidylpeptidase-4 inhibitors (gliptins): Focus on drug–drug interactions. Clin Pharmacokinet 2010; 49: 573–588 [DOI] [PubMed] [Google Scholar]
- 50. Baetta R, Corsini A. Pharmacology of dipeptidyl peptidase-4 inhibitors: Similarities and differences. Drugs 2011; 71: 1441–1467 [DOI] [PubMed] [Google Scholar]
- 51. Scheen AJ. Pharmacokinetics of dipeptidylpeptidase-4 inhibitors. Diabetes Obes Metab 2010; 12: 648–658 [DOI] [PubMed] [Google Scholar]
- 52. Wanner C, Inzucchi SE, Lachin JM et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334 [DOI] [PubMed] [Google Scholar]
- 53. Neal B, Perkovic V, Mahaffey KW et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657 [DOI] [PubMed] [Google Scholar]
- 54. Wiviott SD, Raz I, Bonaca MP et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357 [DOI] [PubMed] [Google Scholar]
- 55. Mosenzon O, Wiviott SD, Cahn A et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: An analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol 2019; 7: 606–617 [DOI] [PubMed] [Google Scholar]
- 56. Perkovic V, Jardine MJ, Neal B et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306 [DOI] [PubMed] [Google Scholar]
- 57. Heerspink HJL, Stefánsson BV, Correa-Rotter R et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436–1446 [DOI] [PubMed] [Google Scholar]
- 58. Marsenic O. Glucose control by the kidney: An emerging target in diabetes. Am J Kidney Dis 2009; 53: 875–883 [DOI] [PubMed] [Google Scholar]
- 59. Rajasekeran H, Kim SJ, Cardella CJ et al. Use of canagliflozin in kidney transplant recipients for the treatment of type 2 diabetes: A case series. Diabetes Care 2017; 40: e75–e76 [DOI] [PubMed] [Google Scholar]
- 60. Schwaiger E, Burghart L, Signorini L et al. Empagliflozin in posttransplantation diabetes mellitus: A prospective, interventional pilot study on glucose metabolism, fluid volume, and patient safety. Am J Transplant 2019; 19: 907–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Attallah N, Yassine L. Use of empagliflozin in recipients of kidney transplant: A report of 8 cases. Transplant Proc 2019; 51: 3275–3280 [DOI] [PubMed] [Google Scholar]
- 62. Halden TAS, Kvitne KE, Midtvedt K et al. Efficacy and safety of empagliflozin in renal transplant recipients with posttransplant diabetes mellitus. Diabetes Care 2019; 42: 1067–1074 [DOI] [PubMed] [Google Scholar]
- 63. Mahling M, Schork A, Nadalin S et al. Sodium–glucose cotransporter 2 (SGLT2) inhibition in kidney transplant recipients with diabetes mellitus. Kidney Blood Press Res 2019; 44: 984–992 [DOI] [PubMed] [Google Scholar]
- 64. Cherney DZ, Kanbay M, Lovshin JA. Renal physiology of glucose handling and therapeutic implications. Nephrol Dial Transplant 2020; 35: i3–i12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Papakitsou I, Vougiouklakis G, Elisaf MS et al. Differential pharmacology and clinical utility of dapagliflozin in type 2 diabetes. Clin Pharmacol 2019; 11: 133–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Filippas-Ntekouan S, Tsimihodimos V, Filippatos T et al. SGLT-2 inhibitors: Pharmacokinetics characteristics and effects on lipids. Expert Opin Drug Metab Toxicol 2018; 14: 1113–1121 [DOI] [PubMed] [Google Scholar]
- 67. Hecking M, Haidinger M, Döller D et al. Early basal insulin therapy decreases new-onset diabetes after renal transplantation. J Am Soc Nephrol 2012; 23: 739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.