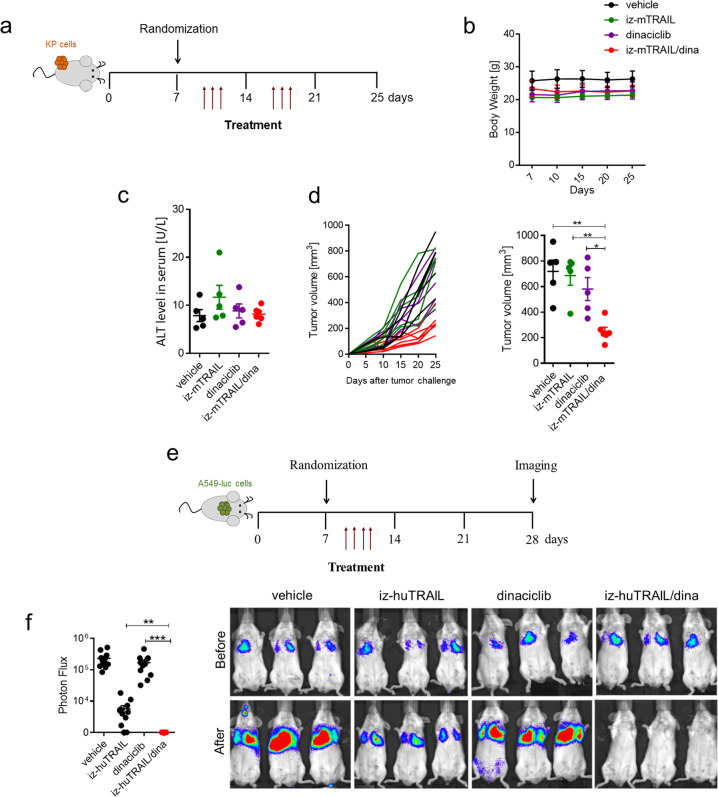
Fig. 3. Co-treatment with TRAIL and CDK9i is well tolerated and exerts therapeutic efficacy in vivo.
a Experimental protocol: iz-mTRAIL (5 mg/kg) and/or dinaciclib (30 mg/kg) or 200 µl buffer were administered intraperitoneally 7 days after subcutaneous injection of 0.5 × 106 KP cells. b Average body weight of KP-bearing mice measured weekly from baseline to 1 week after the end of drug administration. c Serum ALT value 24 h after the last drug administration. d Growth curves of individual KP tumors are shown (left graph) and tumor volume quantification at day 25 (right graph). Dots represent individual mice (n = 5 per group). Error bars represent mean ± SEM. e Experimental protocol: severe combined immunodeficiency (Scid beige) mice were injected with 2 × 106 A549 cells stably expressing luciferase into the lateral tail vein. Seven days after tumor inoculation mice were treated i.p. with iz-huTRAIL (5 mg/kg) and/or dinaciclib (30 mg/kg) or with 200 µl buffer as control for four consecutive days. f Tumor burden was assessed after 28 days via bioluminescence imaging. Dots represent individual mice (n = 11 per group). Error bars represent mean ± SEM.