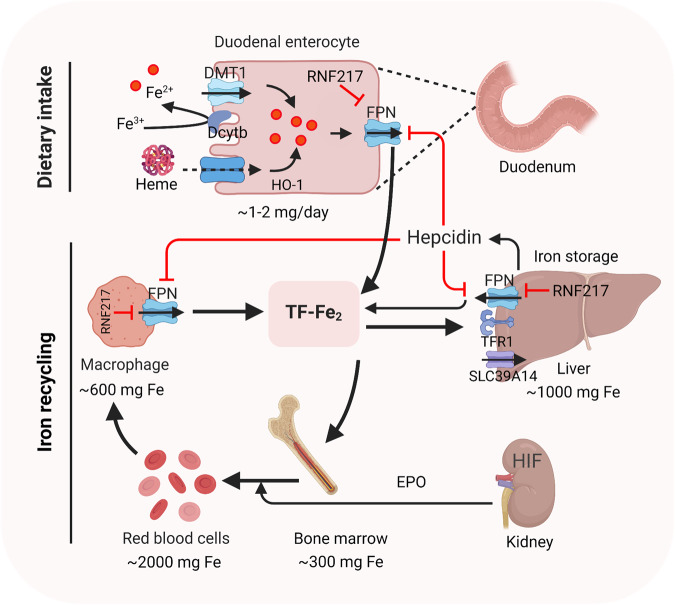
Fig. 2. Regulation of systemic iron homeostasis.
After intake of iron, Fe3+ is reduced by dcytb and then transported into enterocyte through DMT1. Dietary heme is absorpbed by unknown mechanism and degraded in enterocyte by HO-1. Once exported by FPN, Fe3+ binds to transferrin (diferric transferrin, TF-Fe2), travels to tissues, and largely utilized in new red blood cells. Macrophage degraded senescent RBCs to recycle iron. Once needed, EPO, released by kidney, promotes erythropoiesis by HIF signaling pathway. The iron utilization of erythroid marrow and its recycling by macrophages represent the major iron circulation. Excess iron can be stored in hepatocytes through TFR1-mediated TF-Fe2 or SLC39A14-participated non-transferrin-bound iron (NTBI). The release of iron from enterocyte, red blood cells, and macrophages is precisely controlled by FPN, the body’s sole iron exporter, to maintain a relatively stable iron level. The peptide hepcidin, the master regulator of systemic iron homeostasis, is a circulating hormone synthesized by the liver. Recently, we identified RNF217 as a novel E3 ligase for mediating FPN degradation. Dcytb, duodenal cytochrome b; DMT1, divalent metal transporter 1; EPO, erythropoietin; FPN, ferroportin; TFR1, transferrin receptor 1; HO-1, heme oxygenase 1; HIF, hypoxia induced factor; RBCs, red blood cells; NTBI, non-transferrin-bound iron.