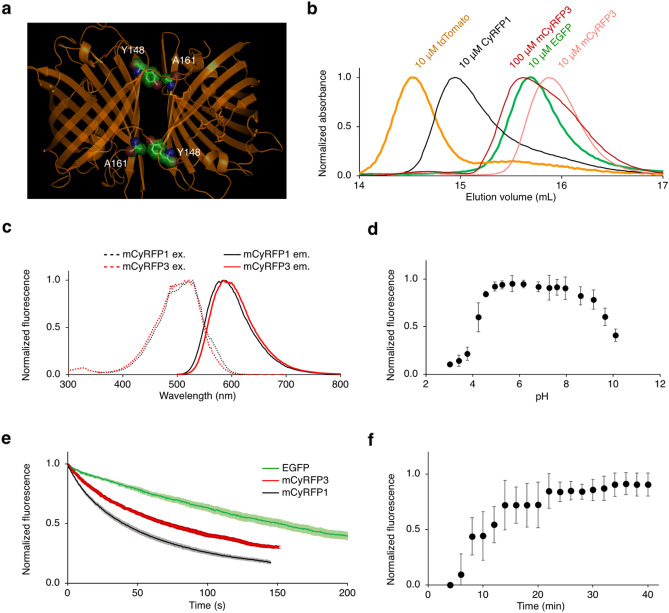
Figure 1.
Engineering and characterization of mCyRFP3. Engineering of mCyRFP3. (a) Crystal structure of dimeric predecessor CyOFP1 displaying interacting residues Y148 and A161. Image was generated in Schrödinger MacPyMol 1.8. (b) Size-exclusion chromatography of EGFP, tdTomato, CyRFP1, and mCyRFP3. EGFP was used as a monomeric standard, while tdTomato was used as a dimeric standard. (c) Excitation and emission spectra of mCyRFP3 compared to its parent mCyRFP1. (d) pH dependence of mCyRFP3 fluorescence, demonstrating a pKa of 4.1. Error bars are s.em.m of triplicate measurements. (e) Photobleaching kinetics of purified cyan- excitable red fluorescent proteins under illumination by a 120-W metal-halide arc lamp through a 490/20-nm excitation filter. The time-axis was adjusted for each fluorophore to simulate excitation conditions producing 1000 photons per s per molecule. Lighter shading on CyRFP1, mCyRFP1, and mCyRFP3 lines represents standard deviation of five measurements. (f) Maturation kinetics of mCyRFP3 demonstrating a half-life of t = 12.5 min. Graphs in (a) through (f) were generated in Microsoft Excel for Mac 16.