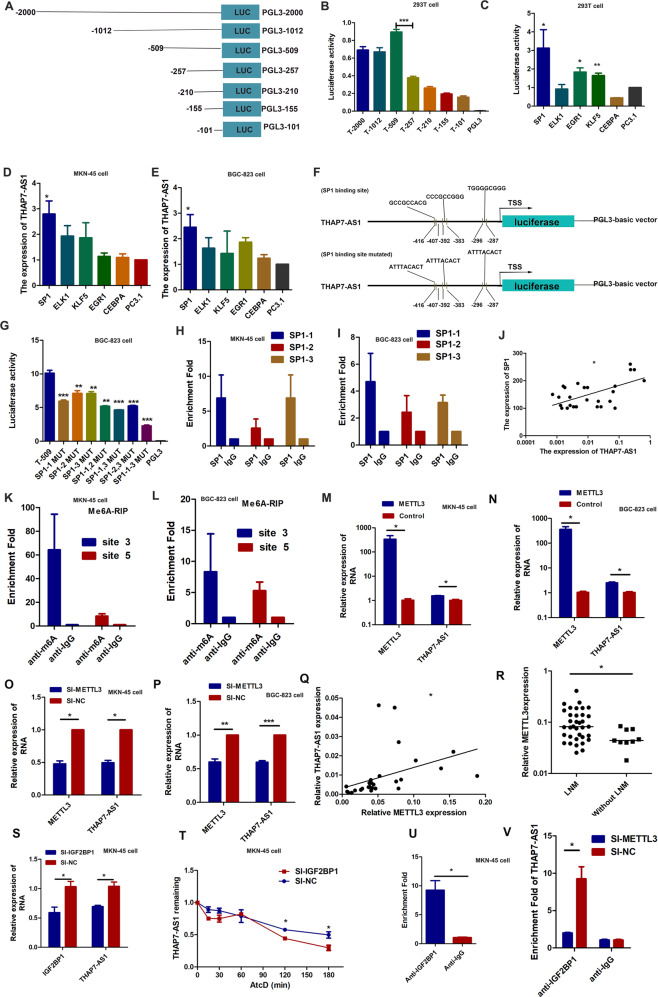
Fig. 2. THAP7-AS1, transcriptionally activated by SP1 and post- transcriptionally stabilized by METTL3-mediated m6A modification.
A Schematic diagram of the THAP7-AS1 promoter fragments spanning from −2,000/ −1,002/ −509/ −257/ −210/ −155/−101 to 0. This promoter fragments were cloned into the upstream of the firefly luciferase reporter gene in the pGL3-basic vector. B Transcriptional activity analysis of the potential THAP7-AS1 promoter fragments in 293T cells. C Luciferase activity assay demonstrated that SP1, EGR1 and KLF5 observably increased promoter activities of pGL3 − 509/0. D–E RT-qPCR assay showed SP1 enhanced the expression level of THAP7-AS1 in MKN-45 cells (D) and BGC-823 cells (E). (F). Three primers that covered the SP1 binding sites at the regions −416 to −407 bp (SP1-1), −392 to −383 bp (SP1-2), −295 to −287 bp (SP1-3) were designed (up). Schematic diagram of the luciferase reporter construct containing the human THAP7-AS1 promoter and the mutant construct (SP1-Mut-1/2/3) containing the basal promoter in which the presumed THAP7-AS1 binding site was mutated (bottom). G Luciferase activity of the THAP7-AS1 promoter was reduced when the three single site (SP1–1, SP1–2 and SP1–3), the two-site (SP1–1,2, SP1–1,3, SP1-2,3) and the three-site mutation (SP1–1-3) were mutated in BGC-823 cells. H–I ChIP-qPCR analysis showed higher fold enrichment of promoter amplicons of SP1 in anti-SP1 antibody group than that of IgG group in MKN-45 cells (H) and BGC-823 cells (I), indicating that SP1 could directly bind to THAP7-AS1 promoter. SP1-1, SP1-2 and SP1-3 respectively represent primer that covered the SP1 binding sites. J A significant positive correlation was found between the protein levels of SP1 and THAP7-AS1 in GC tissues. K–L Enrichment of m6A-modified THAP7-AS1 site 3 and 5 in MKN-45 (K) and BGC-823 cells (L). M–N Transcript levels of METTL3 and THAP7-AS1 in METTL3 and PcDNA3.1 MKN-45 cells (M) and BGC-823 cells (N). O–P Transcript levels of METTL3 and THAP7-AS1 in si-METTL3 and si-NC MKN-45 cells (O) and BGC-823 cells (P). Q A significant positive correlation was found between the METTL3 and THAP7-AS1 in GC tissues. R METTL3 expression was detected in human GC tissues with LNM and without LNM. S Transcript levels of IGF2BP1 and THAP7-AS1 in si-IGF2BP1 and si-NC MKN-45 cells. T MKN-45 cells transfected with SI-IGF2BP1 and control cells treated with Actinomycin D (ActD 5 μg/ml) for the indicated periods of time. THAP7-AS1 levels were analyzed by RT-qPCR. U RNA immunoprecipitation with an anti-IGF2BP1 antibody was used to assess whether IGF2BP1 binding to THAP7-AS1 in MKN-45 cells; IgG was used as the control. V RIP-qPCR using anti-IGF2BP1 antibody showed the affinity of THAP7-AS1 RNA to IGF2BP1 in si-METTL3 cells. *P < 0.05, **P < 0.01, ***P < 0.001.