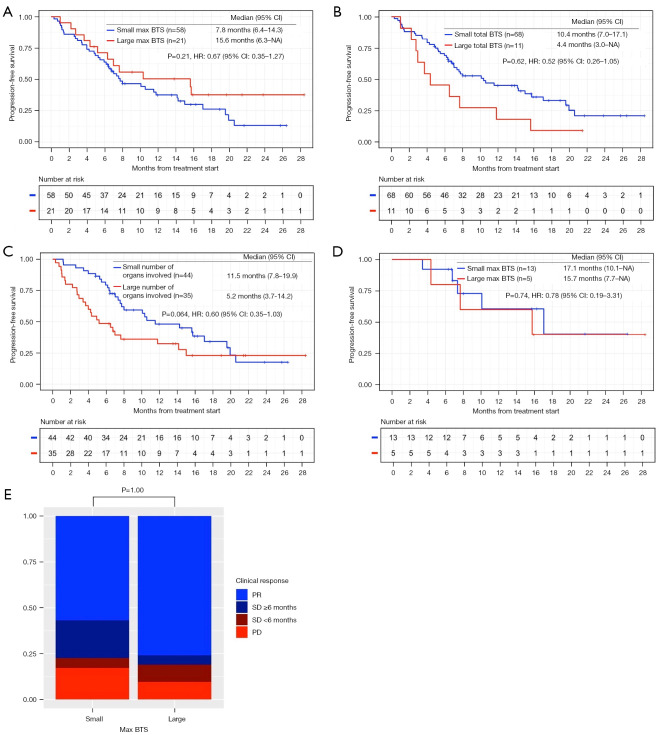
Figure 2.
Clinical outcome of ICI-chemo. (A-D) Kaplan-Meier estimates of the PFS of patients who received ICI-chemo. (A) Comparison between the small and large max BTS groups (n=79). (B) Comparison between the small and large total BTS groups (n=79). (C) Comparison between the small and large number of organs involved groups (n=79). (D) Comparison between the small and large max BTS groups with PD-L1 score ≥50% (n=18). (E) Bar graph showing the percentage of patients with CR, PR SD ≥6 months, SD <6 months, PD. Comparison between the small and large max BTS groups (n=79). ICI, immune-checkpoint inhibitor; ICI-chemo, ICI in combination with chemotherapy; PFS, progression-free survival; BTS, baseline tumor size; max BTS, maximum BTS; PD-L1, programmed death ligand-1; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; HR, hazard ratio; CI, confidence interval.