Abstract
Background
Financial toxicity is a growing problem in oncology, but no prior studies have prospectively measured the financial impact of cancer treatment in a diverse national cohort of newly diagnosed cancer patients. S1417CD was the first cooperative group-led multicenter prospective cohort study to evaluate financial hardship in metastatic colorectal cancer (mCRC) patients.
Methods
Patients aged 18 years or older within 120 days of mCRC diagnosis completed quarterly questionnaires for 12 months. We estimated the cumulative incidence of major financial hardship (MFH), defined as 1 or more of increased debt, new loans from family and/or friends, selling or refinancing home, or 20% or more income decline. We evaluated the association between patient characteristics and MFH using multivariate cox regression and the association between MFH and quality of life using linear regression.
Results
A total of 380 patients (median age = 59.9 years) were enrolled; 77.7% were White, 98.0% insured, and 56.5% had annual income of $50 000 or less. Cumulative incidence of MFH at 12 months was 71.3% (95% confidence interval = 65.7% to 76.1%). Age, race, marital status, and income (split at $50 000 per year) were not statistically significantly associated with MFH. However, income less than $100 000 and total assets less than $100 000 were both associated with greater MFH. MFH at 3 months was associated with decreased social functioning and quality of life at 6 months.
Conclusions
Nearly 3 out of 4 mCRC patients experienced MFH despite access to health insurance. These findings underscore the need for clinic and policy solutions that protect cancer patients from financial harm.
Financial hardship or “financial toxicity” is an increasingly recognized consequence of cancer treatment that results from high out-of-pocket medical costs (eg, copayments and deductibles), nonmedical costs (eg, transportation), and indirect costs (eg, lost work and income). Patients who experience financial hardship during cancer treatment have been shown to be at higher risk for treatment nonadherence, poor quality of life, and worse survival (1-7). The National Cancer Institute (NCI), American Society of Clinical Oncology, and other organizations have advocated for interventions that lessen financial hardship in cancer patients and their families (8–10). However, several gaps in our current understanding of this problem limit the development of highly effective solutions.
Prior studies have estimated that 25%-50% of cancer survivors experience financial hardship (11–15). However, most of these studies have been retrospective and have focused on long-term cancer survivors. Recall bias, particularly with complex personal financial information, is a major limitation. Moreover, these studies may underestimate the incidence and prevalence of financial hardship by not including patients with advanced disease and those on chronic therapy; as survival for diseases like metastatic colorectal cancer increases, so too does the potential financial impact on patients and families. In addition, the cross-sectional design of prior studies limits our understanding of the timing, progression, and potential resolution of treatment-related financial hardship. Such knowledge is critical to the design and implementation of effective interventions. In addition, clinical interventions like financial counseling and navigation will require patients, nonclinical professionals (eg, billing specialists or community-based financial experts), and clinic staff to communicate proactively about cost issues. Normalizing conversations about treatment costs and financial concerns is the first step in building a foundation of trust between patients and clinical teams such that interventions can be implemented successfully. The cancer cooperative groups and NCI Community Oncology Research Program (NCORP) are well poised to take the lead in prioritizing cost-of-care conversations in diverse clinical settings across the United States.
We therefore conducted a longitudinal prospective cohort study (S1417CD) in newly diagnosed metastatic colorectal cancer (mCRC) patients treated at community oncology practices throughout the NCORP network. An overarching goal was to establish the feasibility of prospective collection of financial information from advanced cancer patients, a critical first step on the path toward effective solutions. Our primary study objective was to assess the cumulative incidence of self-reported major financial hardship over a 12-month time horizon.
Methods
Study Design and Eligibility
S1417CD was a prospective cohort study conducted by the SWOG Cancer Research Network. Details of the study design and implementation have been previously reported (16). Patients were enrolled at components and subcomponents of the NCORP, which include more than 1000 community oncology practices throughout the United States. Eligible patients must have been aged 18 years or older and newly diagnosed with stage IV colorectal cancer (de novo or recurrent from an earlier stage diagnosis) within 120 days of registration. Systemic chemotherapy and/or biologic therapy must have been initiated in the 60 days prior to registration or planned within 30 days following registration. Patients receiving supportive or hospice care were not eligible. Patients must have been able to complete questionnaires in English.
Patients were required to give written informed consent in accordance with institutional and federal guidelines (ClinicalTrials.gov identifier: NCT02728804).
Study Questionnaires
Patients completed a self-administered 20-item financial questionnaire following consent (considered the baseline survey) and 27-item follow-up questionnaires at scheduled clinical visits (3, 6, 9, and 12 months) following registration. The questionnaire could be completed in the clinic, at home, or by phone interview. Patients’ caregivers could assist patients with completing questionnaires but were requested not to answer for the patient.
Most items in the comprehensive financial questionnaire were adapted from a questionnaire we previously developed and administered to a population-based sample of patients with stage III colon cancer in the Seattle–Puget Sound region (12). Several questions were also adapted from the Medical Expenditures Panel Survey, a large-scale survey of households, employers, and medical providers on the cost and use of health care in the United States, and the University of Michigan’s Health and Retirement Study, a longitudinal panel survey of older adults (17,18). All questions were modified to ask about financial, employment, and/or insurance changes as they relate to the individual’s cancer diagnosis or treatment costs.
To assess health-related quality of life (HRQOL), we used the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ-C30), which includes 30 items assessing global quality of life, functioning in 5 domains (physical, role, cognitive, emotional, social), and several items assessing specific symptoms (eg, fatigue, trouble sleeping, pain) (19,20). The QLQ-C30 also includes 1 item assessing the impact of medical treatment on finances.
Variable Definitions
Major financial hardship (MFH) was defined as 1 or more of the following during the 12 months following enrollment: accumulating debt of any amount, selling home, refinancing home, experiencing higher than 20% income decline, or borrowing money from family and/or friends. This definition of MFH is consistent with previous studies reporting on financial hardship in cancer patients (11–13). Total assets were the sum of estimated total current value of bank accounts, other financial assets (such as CDs, government bonds, treasury bills), and other properties and assets (eg, second homes and rental properties). Annual household income was defined as the combined total annual income for all household members, from all sources. Prespecified covariates included age (younger than 65 years vs 65 years or older), race (White vs non-White [includes Black, Asian, or Pacific Islander, Other, Unknown] grouped accordingly because of anticipated lower enrollment of minority subjects), marital status (married vs unmarried), employment status (any employment vs unemployed), and income (household income of $50 000 or less per year vs more than $50 000 per year).
Statistical Analysis
Primary Endpoint. The primary aim was to assess the cumulative incidence of MFH. Given serial measurements, the primary endpoint was specified as the time to first evidence of MFH. One-year survival for this population was estimated to be approximately 60% (21–23). Estimates of MFH at 12 months were derived using cumulative incidence to account for the competing risk of death.
Sample Size.The sample size estimate accounted for a 10% dropout for reasons other than death (24). Nondeath-related dropouts were censored. Based on preliminary data, we estimated that 40% of patients would experience MFH in the first year after diagnosis (12). Under this scenario, a sample size (n = 320) of eligible, evaluable patients would allow us to estimate the confidence interval within 8% for an incidence of at least 40%. This estimate assumes no information from the 50% of patients anticipated to drop out. To account for 5% anticipated ineligibility and 10% noncompletion of baseline forms, 374 patients were planned to be enrolled to achieve 320 eligible, evaluable patients.
Specified Secondary Analyses. A key secondary aim was to evaluate whether MFH at 12 months differed by age, race, marital status, employment status, and annual income, categorized as described above. We accounted for multiple comparisons using a Bonferroni method (α = .01) 2-sided test for each comparison. Multivariable Cox regression was used. Per protocol, covariate adjustment for insurance status, education, and sex was included.
We also assessed the relationship between MFH and HRQOL based on the EORTC QLQ-C30. Questionnaire responses were transformed into a linear score (0-100) using the EORTC scoring manual; overall score and scores within each of the 5 domains were determined (20). Using a landmark analysis approach, we categorized patients as having MFH at their 3-month assessment (yes vs no); we then evaluated whether this variable predicted HRQOL (overall score and within each domain) at 6 months using linear regression, including the 3-month HRQOL score as a covariate. We assessed the robustness of potential associations between MFH and HRQOL to large changes in a subset of patients by categorizing HRQOL at 6 months as decline vs no decline, using logistic regression to evaluate the results.
Additional Analyses. Because the accumulation of even small amounts of new debt could be counted as MFH, we examined the robustness of the primary evaluation to changes in the definition of new debt accumulation in sensitivity analyses. Separately, we required that patients have increased 2 debt categories (rather than a single category) to be considered to have experienced MFH because of the accumulation of new debt. Finally, we explored the association of baseline factors with MFH with new debt excluded. In an exploratory unplanned post hoc analysis, we created a simple adverse risk model comprising the variables that were independently associated with MFH. For each patient, we summed up the number of adverse risk factors, creating a score. Cumulative incidence was evaluated by the levels of the risk score. Additionally, in a sensitivity analysis, we also included baseline performance status and treatment type as Cox regression model covariates, as these factors may differentially influence the competing risk of death when comparing financial hardship between groups, potentially generating biased results. Last, because only homeowners are at risk of selling or refinancing a home, we evaluated the cumulative incidence separately for homeowners and nonhomeowners.
Results
Accrual
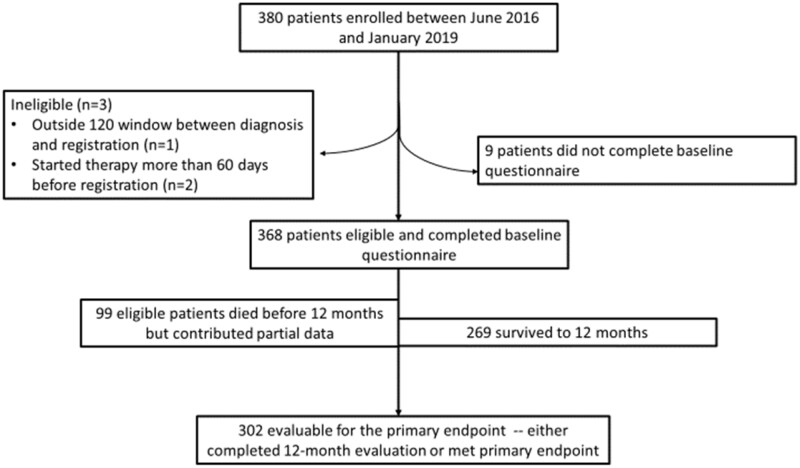
In total, 380 patients were registered between June 2016 and January 2019. The CONSORT flow diagram is shown in Figure 1. Three (0.8%) patients were ineligible: 1 patient was diagnosed with mCRC more than 120 days prior to registration, and 2 started therapy more than 60 days prior to registration. An additional 9 (2.3%) patients did not complete baseline questionnaires and thus were not analyzable. Of the remaining 368 eligible patients with complete baseline questionnaires, 73% were alive at the end of 1 year of follow-up. Among those who died prior to completing the study, partial data—including MFH prior to death—were observed. Thus, 302 (82.1%) patients either reached the primary endpoint as defined in the protocol or had a 12-month evaluation available.
Figure 1.
CONSORT flow diagram.
Patient Characteristics
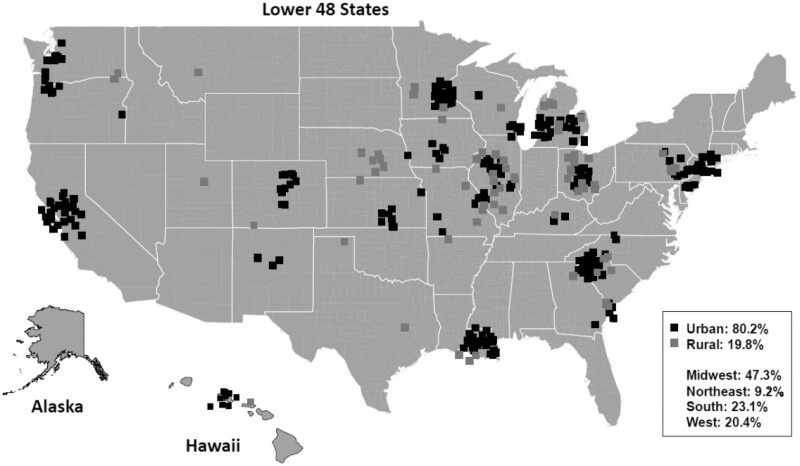
The median age of the cohort was 60.2 years (Table 1). Most (62.8%) patients were younger than 65 years, 61.9% were male, 13.0% were Black, and 56.5% had total household income of $50 000 or less per year. Approximately 60.3% of patients were employed in some capacity prior to diagnosis. Compared with the national colorectal cancer population represented in the Surveillance, Epidemiology, and End Results database (median age = 67 years; 24.6% Black), our study population was slightly younger and had lower representation of Black patients, likely reflecting the challenges in access to clinical research for older and minority populations (25). Enrollments were geographically distributed across the Midwest (47.3%), South (23.1%), West (20.4%), and Northeast (9.2%), with representation in 28 states (Figure 2). Overall, 19.8% of patients were from rural areas, similar to the rate of 19.3% of individuals in the United States from rural areas (26).
Table 1.
Baseline characteristics of eligible patients (n = 368)
| Demographic and clinical characteristics | No. (%) |
|---|---|
| Median age (range), y | 60.2 (21.1-89.3) |
| Age, y | |
| <65 | 231 (62.8) |
| ≥65 | 137 (37.2) |
| Sex | |
| Female | 140 (38.0) |
| Male | 228 (61.9) |
| Race | |
| Asian or Pacific Islander | 17 (4.6) |
| Black | 48 (13.0) |
| White | 286 (77.7) |
| Other or Unknown | 17 (4.6) |
| Marital status | |
| Married/Partnered | 213 (57.9) |
| Divorced or separated | 82 (22.2) |
| Widowed | 17 (4.6) |
| Never married | 48 (13.0) |
| Unknown | 8 (2.2) |
| Primary insurance | |
| Private insurance (employer provided) | 171 (46.5) |
| Medicare | 143 (38.9) |
| Medicaid | 44 (11.9) |
| Other | 3 (0.82) |
| Uninsured | 7 (1.9) |
| Total household income | |
| $0-$25 000 | 114 (30.9) |
| $25 001-$50 000 | 94 (25.5) |
| $50 001-$75 000 | 54 (14.7) |
| $75 001-$100 000 | 31 (8.4) |
| $100 001 or more | 65 (17.7) |
| Unknown | 10 (2.7) |
| Education | |
| High school graduate or less | 143 (38.9) |
| Vocational school or some college | 111 (30.2) |
| Bachelor degree | 63 (17.1) |
| Master, doctorate, or professional degree | 43 (11.7) |
| Missing | 8 (2.2) |
| Homeowner | |
| Yes | 236 (64.1) |
| No | 124 (33.7) |
| Unknown | 8 (2.2) |
| Prediagnosis employment status | |
| Employed (full-time, part-time, self-employed) | 222 (60.3) |
| Retired | 93 (25.3) |
| On leave of absence from paid employment | 2 (0.5) |
| Unemployed | 13 (3.5) |
| Temporary or permanent disability | 25 (6.8) |
| Other or unknown | 13 (3.5) |
| Total assets | |
| $0-$25 000 | 202 (54.9) |
| $25 001-$50 000 | 25 (6.8) |
| $50 001-$100 000 | 38 (10.3) |
| $100 001-$250 000 | 32 (8.7) |
| $250 001-$500 000 | 29 (7.9) |
| $500 001 or more | 34 (9.2) |
| Unknown | 8 (2.2) |
| Prior diagnosis of stage I-III colorectal cancer | |
| Yes | 93 (25.2) |
| No, de novo diagnosis of stage IV | 275 (74.7) |
| ECOG PS | |
| 0 | 183 (49.7) |
| 1 | 158 (42.9) |
| 2 | 22 (5.9) |
| 3 | 4 (1.1) |
| Unknown | 1 (0.3) |
| Initial treatment regimen | |
| (5-FU or cap) ± bevacizumab | 38 (10.3) |
| (FOLFOX or CapOX) ± bevacizumaba | 228 (61.9) |
| (FOLFIRI or CapIri) ± bevacizumaba | 55 (14.9) |
| (FOLFOX, CapOx, FOLFIRI, or CapIRI) + EGFR inhibitor | 15 (4.1) |
| Other | 32 (8.7) |
Exact proportion of patients receiving 5-FU vs capecitabine alone or in the chemotherapy backbone is not measured. 5-FU = 5-fluorouracil; Cap = capecitabine; CapIRI = capecitabine + irinotecan; CAPOX = capecitabine + oxaliplatin; ECOG PS = Eastern Cooperative Oncology Group Performance Status; FOLFIRI = regimen including 5-FU + leucovorin + irinotecan; FOLFOX = regimen including 5-FU + leucovorin + oxaliplatin.
Figure 2.
Geographic distribution of enrollment.
Primary Endpoint
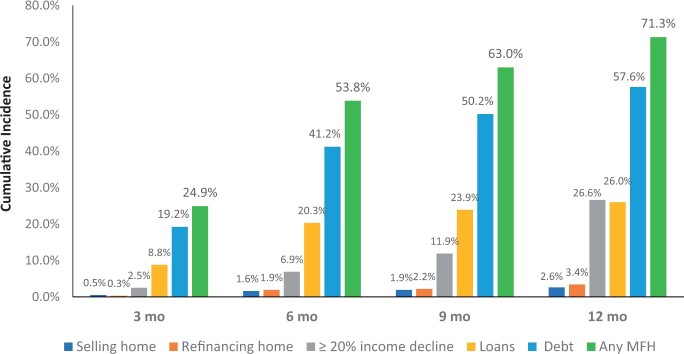
At 12 months, cumulative incidence of MFH was 71.3% (95% confidence interval [CI] = 65.7% to 76.1%) (Figure 3). Of the individual components making up MFH, cumulative incidence at 12 months was 57.6% (95% CI = 51.7% to 63.0%) for new debt, 26.6% (95% CI = 21.3% to 32.0%) for a 20% or more decline in income, 26.0% (95% CI = 21.5% to 30.7%) for new loans from family and/or friends, 3.4% (95% CI = 1.7% to 5.9%) for refinance of home, and 2.6% (95% CI = 1.3% to 4.7%) for sale of home. For many patients, MFH occurred early on, with cumulative incidence estimates of MFH of 24.9% (95% CI = 20.9% to 29.5%), 53.8% (95% CI = 48.5% to 58.8%), and 63.0% (95% CI = 57.8% to 67.8%) at 3, 6, and 9 months, respectively.
Figure 3.
Cumulative incidence of financial hardship. MFH = major financial hardship.
Predictors of Major Financial Hardship
For 4 of the 5 prespecified patient factors (age, race, income, and marital status), there was no statistically significant evidence that MFH differed between groups (Tables 2 and 3). A statistically significantly lower likelihood of MFH was observed among unemployed individuals, although this observation was likely confounded by age and the likelihood that older individuals have greater assets and savings. With new debt excluded from the definition of MFH, similar associations with baseline factors were observed, with the exception that those younger than 65 years were statistically significantly associated with increased risk of MFH (hazard ratio [HR] = 1.71, 95% CI = 1.06 to 2.75; P = .03) and lower total assets were strongly associated with greater likelihood of MFH at all total asset cut points ($25 000, $50 000, and $100 000; Supplementary Tables 1 and 2, available online).
Table 2.
Association of baseline factors and cumulative incidence of major financial hardshipa
| Factor | Cumulative incidence in % of patients (95% CI), % |
|||
|---|---|---|---|---|
| Follow-up assessment time | ||||
| 3 months | 6 months | 9 months | 12 months | |
| Age, y | ||||
| <65 | 44.7 (38.1 to 51.0) | 62.3 (55.6 to 68.2) | 69.9 (63.4 to 75.5) | 73.7 (66.4 to 79.6) |
| ≥65 | 38.2 (30.0 to 46.3) | 52.2 (43.5 to 60.3) | 59.1 (50.3 to 66.9) | 68.1 (58.6 to 76.0) |
| Sex | ||||
| Female | 41.7 (33.4 to 49.8) | 57.6 (48.9 to 65.4) | 66.6 (58.0 to 73.9) | 69.6 (60.8 to 76.7) |
| Male | 42.6 (36.0 to 48.9) | 59.1 (52.3 to 65.2) | 65.4 (58.8 to 71.3) | 72.4 (64.9 to 78.5) |
| Race | ||||
| Non-White | 43.6 (33.5 to 53.2) | 60.4 (49.8 to 69.5) | 68.0 (57.4 to 76.5) | 73.9 (59.4 to 83.9) |
| White | 41.8 (35.8 to 47.6) | 57.9 (51.7 to 63.5) | 65.2 (59.1 to 70.6) | 70.3 (64.1 to 75.6) |
| Marital status | ||||
| Not married or partnered | 42.3 (34.2 to 50.2) | 59.7 (51.2 to 67.2) | 66.2 (57.7 to 73.3) | 69.0 (60.5 to 76.1) |
| Married or partnered | 40.3 (33.6 to 46.8) | 56.4 (49.4 to 62.8) | 64.6 (57.7 to 70.7) | 72.2 (64.0 to 78.8) |
| Income | ||||
| <$50 000/year | 43.8 (36.9 to 50.5) | 59.5 (52.4 to 65.9) | 67.1 (60.1 to 73.1) | 72.7 (65.6 to 78.6) |
| ≥$50 000/year | 38.0 (30.2 to 45.7) | 56.0 (47.6 to 63.5) | 63.5 (55.2 to 70.7) | 69.1 (59.3 to 77.1) |
| <$100 000/year | 44.4 (38.6 to 50.0) | 60.3 (54.4 to 65.7) | 67.9 (62.1 to 73.0) | 72.9 (67.0 to 77.9) |
| ≥$100 000/year | 27.7 (17.4 to 38.9) | 47.7 (35.0 to 59.3) | 55.5 (42.4 to 66.7) | 64.9 (45.6 to 78.8) |
| Education | ||||
| ≤High school/GED | 43.1 (34.8 to 51.1) | 55.9 (47.3 to 63.7) | 63.1 (54.5 to 70.5) | 72.1 (62.3 to 79.7) |
| >High school | 41.7 (35.2 to 48.1) | 60.2 (53.4 to 66.3) | 67.7 (61.0 to 73.4) | 70.4 (63.6 to 76.2) |
| Insurance status | ||||
| Suboptimal insuranceb | 39.4 (28.0 to 50.6) | 54.9 (42.5 to 65.7) | 67.8 (55.3 to 77.5) | 72.4 (59.2 to 82.0) |
| Private/Medicare/military | 42.9 (37.2 to 48.5) | 59.4 (53.5 to 64.8) | 65.4 (59.6 to 70.6) | 70.8 (64.6 to 76.2) |
| Medicare | 37.4 (29.5 to 45.4) | 52.5 (43.8 to 60.4) | 58.5 (49.8 to 66.2) | 64.7 (55.6 to 72.5) |
| Non-Medicare | 45.3 (38.6 to 51.7) | 62.3 (55.6 to 68.3) | 70.5 (64.0 to 76.0) | 75.8 (68.1 to 81.9) |
| Medicaid | 50.0 (34.3 to 63.8) | 65.9 (49.5 to 78.1) | 77.6 (61.3 to 87.7) | 85.4 (66.9 to 94.0) |
| Non-Medicaid | 41.2 (35.8 to 46.5) | 57.5 (51.9 to 62.7) | 64.3 (58.7 to 69.3) | 69.3 (63.3 to 74.5) |
| Uninsured | 55.6 (17.5 to 82.0) | 55.6 (17.5 to 82.0) | 77.8 (28.1 to 95.1) | 77.8 (28.1 to 95.1) |
| Insured | 41.9 (36.7 to 47.0) | 58.6 (53.3 to 63.5) | 65.6 (60.3 to 70.3) | 71.1 (65.4 to 76.0) |
| Total assets | ||||
| <$25 000 | 47.1 (40.0 to 53.9) | 63.5 (56.3 to 69.8) | 69.3 (62.2 to 75.3) | 75.0 (66.8 to 81.5) |
| ≥$25 000 | 33.7 (26.5 to 41.1) | 50.6 (42.6 to 58.1) | 60.3 (52.2 to 67.5) | 65.7 (57.2 to 72.9) |
| <$50 000 | 47.3 (40.6 to 53.6) | 62.4 (55.7 to 68.4) | 68.3 (61.8 to 74.0) | 74.8 (67.2 to 80.9) |
| ≥$50 000 | 30.5 (22.8 to 38.5) | 49.6 (40.7 to 57.9) | 59.9 (50.9 to 67.8) | 64.0 (54.7 to 71.9) |
| <$100 000 | 46.7 (40.5 to 52.6) | 62.8 (56.6 to 68.3) | 68.6 (62.6 to 73.9) | 74.9 (68.4 to 80.4) |
| ≥$100 000 | 25.5 (17.2 to 34.7) | 43.6 (33.4 to 53.4) | 55.7 (44.9 to 65.2) | 58.9 (47.6 to 68.5) |
| Employment | ||||
| Unemployed | 36.0 (28.1 to 43.9) | 51.0 (42.4 to 58.9) | 57.7 (49.0 to 65.4) | 62.9 (53.8 to 70.7) |
| Any employmentc | 46.2 (39.5 to 52.6) | 63.3 (56.5 to 69.2) | 71.1 (64.6 to 76.6) | 76.2 (69.2 to 81.9) |
| Employment (<65 years) | ||||
| Unemployed | 38.2 (24.0 to 52.3) | 58.8 (42.6 to 71.9) | 65.7 (49.3 to 78.0) | 65.7 (49.3 to 78.0) |
| Any employmentc | 46.2 (38.8 to 53.2) | 63.1 (55.6 to 69.6) | 70.9 (63.7 to 77.0) | 75.5 (67.2 to 82.0) |
| Homeowner | ||||
| No | 42.3 (33.4 to 50.8) | 60.2 (50.9 to 68.3) | 66.1 (56.9 to 73.8) | 71.8 (62.3 to 79.2) |
| Yes | 40.5 (34.2 to 46.7) | 56.4 (49.8 to 62.5) | 64.7 (58.2 to 70.5) | 69.9 (62.8 to 76.0) |
Financial hardship that includes all protocol-specified components including new debt accumulation. CI = confidence interval; GED = general education development.
Defined as including Medicaid (including “dual eligible” patients with Medicare + Medicaid) or no insurance.
Includes full-time employment, part-time employment, self-employed, and on leave from full-time employment.
Table 3.
Association of baseline factors and risk of major financial hardshipa
| Factor | Multivariate Cox regressiona |
|
|---|---|---|
| Hazard ratio (95% CI) | P b | |
| Age | ||
| <65 years vs ≥65 years | 0.93 (0.66 to 1.29) | .65 |
| Sex | ||
| Female vs male | 0.88 (0.67 to 1.16) | .36 |
| Race | ||
| Non-White vs White | 1.06 (0.79 to 1.43) | .70 |
| Marital status | ||
| Not married or partnered vs married or partnered | 0.98 (0.73 to 1.31) | .87 |
| Income per year | ||
| <$50 000 vs ≥$50 000 | 1.33 (0.95 to 1.87) | .10 |
| <$100 000 vs ≥$100 000 | 1.92 (1.28 to 2.89) | .002 |
| Education | ||
| ≤High school/GED vs >high school | 0.89 (0.67 to 1.18) | .41 |
| Insurance status | ||
| Suboptimal insurancec vs private/Medicare/military |
0.74 (0.51 to 1.07) | .11 |
| Medicare vs non-Medicare | 0.96 (0.63 to 1.47) | .87 |
| Medicaid vs non-Medicaid | 1.09 (0.72 to 1.65) | .67 |
| Uninsured vs insured | 0.87 (0.39 to 1.94) | .73 |
| Total assets | ||
| <$25 000 vs ≥$25 000 | 1.28 (0.93 to 1.76) | .12 |
| <$50 000 vs ≥$50 000 | 1.30 (0.95 to 1.77) | .10 |
| <$100 000 vs ≥$100 000 | 1.57 (1.12 to 2.20) | .009 |
| Employment | ||
| Unemployed vs any employmentd | 0.66 (0.48 to 0.92) | .01 |
| Employment (<65 years) | ||
| Unemployed vs any employmentd | 0.69 (0.43 to 1.09) | .11 |
| Homeowner | ||
| No vs yes | 0.99 (0.74 to 1.33) | .94 |
Primary multivariable model includes indicators for age younger than 65 years, female sex, non-White race, unmarried or unpartnered, income <$50 000 per year, ≤high school/GED, suboptimal insurance, total assets <$100 000, unemployed, nonhomeowner. For estimating employment effect in those younger than 65 years, the primary model (excluding the age indicator) is run on the subset of younger than 65 years. For estimating hazard ratio of other cut points within the variables of interest, a model using the same indicators as in the primary model is used, with an indicator for the new cut point/variable replacing the primary model’s indicator in the same category. CI = confidence interval; GED = general education development.
P value calculated using χ2 test, Bonferroni α = .01; 2-sided test was used for each comparison.
Defined as including Medicaid (including “dual eligible” patients with Medicare + Medicaid) or no insurance.
Includes full-time employment, part-time employment, self-employed, and on leave from full-time employment.
The relationship between prespecified variables categorized differently and additional baseline variables and MFH were examined (Table 3). Patients with a household income of less than $100 000 per year and patients with total assets of less than $100 000 per year were at increased observed risk of MFH.
Major Financial Hardship and HRQOL
Patients with MFH at 3 months, compared with those without, reported lower scores on the EORTC functional scales at 6 months, adjusting for quality of life at 3 months, although only the Social Functioning score (average drop of 9.1 points; P = .002) and the Global Health Status (Overall QOL) score (average drop of 4.2 points; P = .03) were statistically significant (Table 4). Social functioning remained statistically significant in the logistic regression model examining any drop (yes vs no) in functioning, whereas global health status was no longer statistically significant.
Table 4.
Association of MFH at 3 months with quality of life (EORTC QLQ-C30) scores at 6 months
| Domain | Linear regression (continuous score) |
Logistic regression (any drop) |
||
|---|---|---|---|---|
| Linear estimatea (SE) | P b | OR estimatea (95% CI) | P c | |
| Physical functioning | −1.7 (2.0) | .39 | 0.91 (0.55 to 1.52) | .72 |
| Role functioning | −4.9 (2.9) | .09 | 0.58 (0.33 to 1.02) | .06 |
| Cognitive functioning | −2.0 (2.0) | .30 | 1.00 (0.56 to 1.80) | .99 |
| Emotional functioning | −0.8 (2.0) | .70 | 0.82 (0.48 to 1.41) | .47 |
| Social functioning | −9.1 (2.9) | .002 | 0.56 (0.32 to 0.99) | .05 |
| Overall QOL (total score) | −4.2 (1.9) | .03 | 0.76 (0.45 to 1.30) | .32 |
Estimate of effect of financial toxicity at 3 months on 6-month quality of life (QOL) index score, controlled for patient’s QOL index score at 3 months. CI = confidence interval; EORTC QLQ-C30 = European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire; MFH = major financial hardship; OR = odds ratio.
P value calculated using t tests, 2-sided α = .05.
P value calculated using χ2 test, 2-sided α = .05.
Additional Analyses
In the sensitivity analysis omitting the new debt measure, the cumulative incidence of MFH at 1 year was 43.0% (95% CI = 37.3% to 48.6%). With an increase of 2 debt categories required to consider patients to have accumulated new debt, 32.7% (95% CI = 27.8% to 37.7%) of patients were estimated to have new debt by 1 year, and the overall estimate of MFH at 1 year was 58.2% (95% CI = 52.5% to 63.6%).
In an exploratory post hoc analysis, income less than $100 000 and total assets less than $100 000 were both adversely associated with MFH. Each increase in the number of these 2 risk factors from 0 to 1 and 1 to 2 was associated with a 49% increased risk of MFH (HR = 1.49, 95% CI = 1.21 to 1.85; P < .001). The inclusion of baseline performance status and treatment in the Cox regression analyses comparing the cumulative incidence of MFH for different patient groups had minimal influence on the results (Supplementary Table 3). The 12-month cumulative incidence of MFH was very similar for homeowners (69.9%, 95% CI = 62.8% to 76.0%) and nonhomeowners (71.8%, 95% CI = 62.3% to 79.2%).
Discussion
In a prospective study of financial outcomes in a diverse cohort of insured mCRC patients treated at community oncology sites throughout the country, we found that the cumulative incidence of financial hardship increased consistently over time, such that nearly two-thirds of patients faced MFH within 1 year of initiating treatment. Though we did see a trend toward increased risk of MFH in younger, non-White, and lower income patients, we did not find a statistically significant association between any of these patient factors and MFH, suggesting that MFH is a common occurrence across these key categories. In an exploratory post hoc analysis, we also observed that individuals with annual incomes of less than $100 000 and total assets of less than $100 000 had more than twice the risk of MFH than individuals with neither factor, a finding consistent with the hypothesis that patients with limited resources are much more susceptible to the devastating financial impact of a cancer diagnosis.
We also found that MFH precedes decrements in HRQOL. A conceptual model developed by Yabroff and Tucker-Seeley categorizes financial hardship as material conditions (eg, debt), psychological responses (eg, financial worry), and coping behaviors (eg, cost-related nonadherence) (6,27,28). Our finding that patients who experience MFH at 3 months were more likely to experience subsequent declines in social functioning and overall quality of life suggest that the material and psychological experiences of financial hardship are associated.
We chose to focus on mCRC to minimize the heterogeneity in treatments and associated costs in our study population and because we hypothesize that increases in mCRC survival have also led to increased financial burdens because of the chronic and intensive nature of treatment. Our observation that more than 70% of eligible mCRC patients survived to 1 year, although encouraging, also suggests that addressing families’ financial concerns is increasingly important, particularly because nearly 75% of 1-year survivors experienced MFH. In addition, given that approximately one-quarter and one-half of patients experienced MFH at 3 months and 6 months, respectively, financial concerns need to be addressed as close to diagnosis as possible.
Our findings also suggest that interventions to relieve cancer-related financial toxicity may be broadly applicable to most cancer patients. Given that 98% of the cohort had health-care insurance, our findings can inform the national policy and payer discussion regarding health insurance and underinsurance in the United States. Additionally, interventions that help patients access assistance resources for nonmedical costs, navigate employment benefits, and manage their other life expenses in the context of cancer diagnosis are needed. Given that financial hardships are experienced early and accumulate quickly during the first year after diagnosis, such interventions should be deployed at diagnosis and throughout the care continuum.
Finally, our experience with accrual shows that patients and families believe that financial toxicity is an important issue worth studying. Despite initial concern from sites and investigators that patients would be hesitant to participate, we completed enrollment more quickly than we had anticipated, in part because of efforts by NCORP sites, investigators, and patient advocates to address patients’ questions around privacy and data security and explain the study’s larger purpose (16). That metastatic cancer patients were willing to share sensitive financial information bodes well for patient engagement with future interventional studies.
In interpreting our study findings, several limitations should be acknowledged. First, although we focused on a 12-month time horizon, patients who survive beyond 12 months may experience further financial deterioration. Future studies should examine financial issues at end of life, when financial hardship may also be associated with more aggressive use of care (29). Next, given that enrollment could occur within 120 days after a mCRC diagnosis, some baseline assessments may not reflect patient financial status at diagnosis. Although ideally we would have enrolled and surveyed patients immediately after diagnosis, such an approach presented challenges to enrollment feasibility, and the study team chose to allow this eligibility criterion to be more inclusive. Further, though we asked patients to report on financial changes they experienced specifically as a result of cancer diagnosis and treatment-related costs, attribution of the experience of MFH to cancer is likely uncertain in some cases. In particular, some patients, particularly those with tenuous finances before cancer, would have inevitably faced these hardships even in the absence of cancer. Next, our definition of MFH was highly sensitive to accrual of new debt. We chose not to set a specific debt amount, based on the premise that any experience of debt suggests lack of savings or liquid assets. Nonetheless, even with new debt excluded from the definition of MFH, a substantial proportion of patients experienced MFH. Additionally, we found evidence that risk of MFH is cumulative across levels of income and total assets. Although this finding was derived from an unplanned post hoc analysis, it nonetheless suggests that risk of MFH may be cumulative across patient factors and provides the predicate for future analyses using this cohort to examine whether a multidomain risk prediction model for financial hardship can be derived. Finally, our study findings may not be fully generalizable to the real-world mCRC population, which tends to be older and more diverse. Because of limited resources and issues of data privacy for nonconsenting individuals, we were unable to obtain data on patients who were screened but did not enroll, which could illuminate patterns of selection bias for trial participants, a common challenge in the conduct of trials (30–32). Still, we believe that enrollment from a national sample of community clinical practices with wide geographic distribution makes our findings more generalizable than most previously published studies on financial hardship.
In summary, our study findings draw attention to deficiencies in the US health-care system and economic safety nets that are unable to prevent the majority of cancer patients from experiencing financial hardship. Policy solutions that improve access to affordable health care and insurance benefit designs that minimize cost sharing for evidence-based cancer treatments are examples of strategies that can mitigate financial hardship. At the clinic level, interventions that connect patients and caregivers with financial counseling, assistance, and navigation resources are critical. Building on our initial insights from this study, we are actively analyzing credit data collected in this study to identify patient groups particularly vulnerable to MFH so that interventions can be targeted and tailored to their needs.
Funding
This work was supported by the ASCO Foundation Conquer Cancer Career Development Award 2013, SWOG Hope Foundation Charles Coltman Jr Award (2010), and by National Cancer Institute of the National Institutes of Health grant awards UG1CA189974, U10CA180820, U10CA180821, and U10CA180868.
Notes
Role of the funders: The funders did not play a role in any of the following: the design of the study, data collection, data analysis, data interpretation, manuscript writing, and decision to submit the manuscript for publication. The ASCO Foundation Conquer Cancer Career Development Award 2013 and SWOG Hope Foundation Charles Coltman Jr Award (2010) provided institutional salary support and academic travel support to Dr Shankaran to develop this research study as well as funding for access to credit report data. The SWOG NCI NCORP Research Base grant, UG1CA189974, provided administrative support for the conduct of the trial. U10CA180820, U10CA180821, and U10CA180868 (ECOG-ACRIN, Alliance and NRG Oncology) provided support for Ochsner Cancer Institute to participate in this trial.
Disclosures: None of the authors have relevant relationships to disclose.
Author contributions: Conceptualization: Shankaran, Hershman, Ramsey, Unger. Data Curation: Unger, Darke. Formal Analysis: Shankaran, Unger, Darke, Ramsey, Hershman. Funding Acquisition: Shankaran. Investigation: Shankaran, Unger, Darke, Suga, Wade, Kourlas, Chandana, O’Rourke, Satti, Liggett, Hershman, Ramsey. Methodology: Shankaran, Unger, Darke, Ramsey, Hershman. Project administration: Shankaran, Liggett, Ramsey, Hershman. Resources: Suga, Wade, Kourlas, Chandana, O’Rourke, Satti. Methodology: Shankaran, Unger, Darke, Ramsey, Hershman. Software: Unger, Darke. Supervision: Shankaran. Validation: Shankaran, Unger, Darke, Ramsey, Hershman. Visualization: Shankaran, Unger, Darke, Ramsey, Hershman. Writing (original draft): Shankaran, Unger, Darke, Ramsey, Hershman. Writing (review and editing): Shankaran, Unger, Darke, Suga, Wade, Kourlas, Chandana, O’Rourke, Satti, Liggett, Hershman, Ramsey.
Disclaimers: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Prior presentations: Portions of this manuscript have been presented at 2020 ASCO Annual Meeting and 2020 ASCO Quality Care Symposium.
Data Availability
The data underlying this article cannot be shared publicly due to protection of privacy of individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.
Supplementary Material
References
- 1. Banegas MP, Schneider JL, Firemark AJ, et al. The social and economic toll of cancer survivorship: a complex web of financial sacrifice. J Cancer Surviv. 2019;13(3):406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caram MEV, Oerline MK, Dusetzina S, et al. Adherence and out-of-pocket costs among Medicare beneficiaries who are prescribed oral targeted therapies for advanced prostate cancer. Cancer. 2020;126(23):5050–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jazowski SA, Dusetzina SB.. Addressing cost-related nonadherence to oral anticancer medications through health policy reform: challenges and opportunities. Cancer. 2020;126(16):3613–3616. [DOI] [PubMed] [Google Scholar]
- 4. Ramsey SD, Bansal A, Fedorenko CR, et al. Financial insolvency as a risk factor for early mortality among patients with cancer. J Clin Oncol. 2016;34(9):980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rotter J, Spencer JC, Wheeler SB.. Financial toxicity in advanced and metastatic cancer: overburdened and underprepared. J Oncol Pract. 2019;15(4):e300–e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tucker-Seeley RD, Thorpe RJ.. Material-psychosocial-behavioral aspects of financial hardship: a conceptual model for cancer prevention. Gerontologist. 2019;59(suppl 1):S88–S93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Winn AN, Keating NL, Dusetzina SB.. Factors associated with tyrosine kinase inhibitor initiation and adherence among Medicare beneficiaries with chronic myeloid leukemia. J Clin Oncol. 2016;34(36):4323–4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dusetzina SB, Ramsey SD, Shankaran V, Yabroff KR. Financial Toxicity (Financial Distress) and Cancer Treatment (PDQ(R)): Patient Version. PDQ Cancer Information Summaries. Bethesda, MD; 2019.
- 9. Meropol NJ, Schrag D, Smith TJ, et al. ; for the American Society of Clinical Oncology. American Society of Clinical Oncology guidance statement: the cost of cancer care. J Clin Oncol. 2009;27(23):3868–3874. [DOI] [PubMed] [Google Scholar]
- 10. Schnipper LE. ASCO task force on the cost of cancer care. J Oncol Pract. 2009;5(5):218–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Banegas MP, Guy GP Jr, de Moor JS, et al. For working-age cancer survivors, medical debt and bankruptcy create financial hardships. Health Aff (Millwood). 2016;35(1):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shankaran V, Jolly S, Blough D, Ramsey SD.. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer: a population-based exploratory analysis. J Clin Oncol. 2012;30(14):1608–1614. [DOI] [PubMed] [Google Scholar]
- 13. Yabroff KR, Dowling EC, Guy GP Jr, et al. Financial hardship associated with cancer in the United States: findings from a population-based sample of adult cancer survivors. J Clin Oncol. 2016;34(3):259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yabroff KR, Zhao J, Han X, Zheng Z.. Prevalence and correlates of medical financial hardship in the USA. J Gen Intern Med. 2019;34(8):1494–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zheng Z, Jemal A, Han X, et al. Medical financial hardship among cancer survivors in the United States. Cancer. 2019;125(10):1737–1747. [DOI] [PubMed] [Google Scholar]
- 16. Shankaran V, Unger JM, Darke AK, Hershman DL, Ramsey SD.. Design, data linkage, and implementation considerations in the first cooperative group led study assessing financial outcomes in cancer patients and their informal caregivers. Contemp Clin Trials. 2020;95:106037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.HRS: Health and Retirement Study; 2020. https://hrs.isr.umich.edu/welcome-health-and-retirement-study. Accessed January 6, 2020.
- 18.Agency for Healthcare Research and Quality. Medical expenditure panel survey; 2020. https://www.meps.ahrq.gov/mepsweb/. Accessed January 6, 2020. [PubMed]
- 19. Aminisani N, Nikbakht H, Asghari Jafarabadi M, Shamshirgaran SM.. Depression, anxiety, and health related quality of life among colorectal cancer survivors. J Gastrointest Oncol. 2017;8(1):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.EORTC. Quality of Life. version 3. https://www.eortc.org/app/uploads/sites/2/2018/08/Specimen-QLQ-C30-English.pdf. Published 1995. Accessed January 6, 2020.
- 21. Shi Q, de Gramont A, Grothey A, et al. Individual patient data analysis of progression-free survival versus overall survival as a first-line end point for metastatic colorectal cancer in modern randomized trials: findings from the analysis and research in cancers of the digestive system database. J Clin Oncol. 2015;33(1):22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Renfro LA, Goldberg RM, Grothey A, et al. ; for the ARCAD Clinical Trials Program. Clinical calculator for early mortality in metastatic colorectal cancer: an analysis of patients from 28 clinical trials in the Aide et Recherche en Cancerologie Digestive Database. J Clin Oncol. 2017;35(17):1929–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sanford NN, Ahn C, Beg MS, et al. Stage-specific conditional survival among young (age below 50 y) versus older (age 50 y and above) adults with colorectal cancer in the United States. Am J Clin Oncol. 2020;43(7):526–530. [DOI] [PubMed] [Google Scholar]
- 24. Biazevic MGH, Antunes JLF, Togni J, de Andrade FP, de Carvalho MB, Wünsch-Filho V.. Survival and quality of life of patients with oral and oropharyngeal cancer at 1-year follow-up of tumor resection. J Appl Oral Sci. 2010;18(3):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Cancer stat facts: colorectal cancer; 2021. https://seer.cancer.gov/statfacts/html/colorect.html. Accessed July 17, 2021.
- 26.US Census Bureau. Measuring America: our changing landscape; 2016. https://www.census.gov/content/dam/Census/library/visualizations/2016/comm/acs-rural-urban.pdf. Accessed August 3, 2021.
- 27. Tucker-Seeley RD, Yabroff KR.. Minimizing the “financial toxicity” associated with cancer care: Advancing the research agenda. J Natl Cancer Inst. 2016;108(5):djv410. [DOI] [PubMed] [Google Scholar]
- 28. Zheng Z, Jemal A, Tucker-Seeley R, et al. Worry about daily financial needs and food insecurity among cancer survivors in the United States. J Natl Compr Canc Netw. 2020;18(3):315–327. [DOI] [PubMed] [Google Scholar]
- 29. Tucker-Seeley RD, Abel GA, Uno H, Prigerson H.. Financial hardship and the intensity of medical care received near death. Psychooncology. 2015;24(5):572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS.. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–2067. [DOI] [PubMed] [Google Scholar]
- 31. Unger JM, Barlow WE, Martin DP, et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. 2014;106(3):dju002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Unger JM, Coltman CA Jr, Crowley JJ, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol. 2006;24(1):141–144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to protection of privacy of individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.