Abstract
Cartilage defects are one of the hardest injures to cure, given the limited regenerative ability of cartilage tissues. Moreover, cartilage defects affect an increasing number of people worldwide. Therefore, scientists have attempted to develop effective strategies to repair cartilage defects in recent years. Recent advances in tissue engineering have led to the strategies for inducing cartilage regeneration. Among the emerging strategies, scaffolds are commonly used in cartilage tissue engineering (CTE) as they provide favorable environment for the growth and proliferation of chondrocytes. An ideal scaffolding material should be highly biocompatible. Type I collagen is one such material, which is widely used in CTE. However, type I collagen has poor mechanical properties and stability, which limit its use. Cross-linking is a simple method known to improve degradability, biological and mechanical properties of biomaterials by enhancing chemical and physical interactions between polymers. Cross-linking can be induced through chemical, physical or biological processes. In this review, we present cross-linking methods that can enhance the mechanical strength of type I collagen for CTE and highlight future directions in this field.
Keywords: Type I collagen, cross-linking, tissue engineering, biomaterials, cartilage
Introduction
The incidence of cartilage defects, such as articular cartilage defect, ear cartilage defect, and costal cartilage defect has been on the rise worldwide. The main treatment strategies for cartilage defects mainly include palliative treatment with arthroscopic lavage and debridement, restorative treatment with bone marrow stimulation techniques, and tissue engineering approaches such as autologous chondrocyte implantation and osteochondral transplantation [1]. Total joint arthroplasty or osteochondral allograft is the main interventions for severe cartilage defects. The treatment and care of patients with cartilage defects is associated with high psychological stress and financial cost. Cartilage tissue has high differentiation rate, lacks nerves, vessels, lymph and has limited regenerative ability [2]. Therefore, the repair of damaged cartilage is challenging owing to these features [3,4]. Advances in cartilage tissue engineering (CTE) have led to the development of different methods for cartilage regeneration [5,6]. Cartilage is made of chondrocytes (a single cell-type), which makes it easier to repair cartilage through CTE. Cartilage tissue engineering is a new field whereby cells are seeded on biomaterials with good biocompatibility and biodegradability to form a complex and then the complex is implanted to the cartilage defect site to form new cartilage [7] (Figure 1). Effective construction of CTE material requires three elements: (1) suitable scaffold, (2) sufficient seed cells with normal function, (3) cytokines for regulation of cell proliferation and maintenance of cell phenotypic characteristics [8]. Scaffold is key component of CTE as it provides a three-dimension environment in which cells can grow, proliferate, perform normal function and protect them from harmful factors [9,10]. Therefore, the scaffold should effectively release bioactive molecules/drugs, undergo degradation in a controlled manner, undergo sterilization without losing its bioactivity, and should not cause chronic inflammatory reactions or produce toxic degradation products.
Figure 1.

The process of cartilage tissue engineering. Cartilage cells are extracted and expanded through culture and grown on a scaffold for in vitro culture. The cells are then implanted into the human body over a period of time to treat cartilage defects (such as ear cartilage defects and articular cartilage defects).
Collagen is the primary structural protein of human tissues, and it provides physical support to tissues and plays an important role in maintaining the structural and biological integrity of the extracellular matrix (ECM) [11,12]. Collagen can be harvested from a wide range of sources such as the skeleton, cartilage, skin, nerve, vessel and tendon [13]. Collagen, mainly the type I collagen is a promising biomaterial applied in CTE owing to its high biocompatibility, biodegradability, low immunogenicity and availability of several sources [3,14]. Type I collagen accounts for approximately 80-90% of the total collagen content and is the most widely used type of collagen in medicine [15]. In this review, type I collagen is used as a representative scaffold material to explore the application of collagen in CTE. The sources and structural characteristics of type I collagen are also discussed. Application of composite scaffolds and use of cross-linkers to overcome the several limitations such as poor mechanical strength and rapid degradation of scaffolds based on type I collagen are also described. Cross-linkers constitute the main approach used for production of composite scaffolds. This cross-linking effect is achieved by changing the chemical bonds within the type I collagen molecule. Many amino acids such as glutamate, asparagine, lysine, etc. in type I collagen participate in the cross-linking process. The methods used for type I collagen cross-linking are divided into physical cross-linking, chemical cross-linking and biological cross-linking. The principles, advantages and disadvantages of these cross-linking reactions are different. Therefore, the role and classification of cross-linkers for type I collagen scaffolds are discussed in detail in the review.
Type I collagen
Mammalian collagen comprises three-quarters of the dry weight of skin, and accounts for one-third of total protein. Moreover, it is the most ubiquitous ECM component in mammals [16]. Approximately 28 types of collagens have been identified [17], with type I collagen being a major type of total collagen. Collagen has a length of 2800 Å, a diameter of 14-15 Å and a molecular weight of approximately 300,000 Da. Collagen comprises three α chains organized in a triple helix conformation, characterized by a repeating sequence (Gly-X-Y)n. The X and Y positions in the motif mainly comprise a proline residue and contribute to formation of the triple helix conformation by restricting the dihedral angle of the main chain [18]. Type I collagen is derived from a wide variety of sources. It is an ideal biomaterial for CTE owing to its good biocompatibility, biodegradability, ability to be combined with other biomaterials, good cell adhesion properties, hydrophilicity and low antigenicity [19,20].
Source and extraction methods of type I collagen
Type I collagen can be harvested from mammals, amphibians, fishes, marines and avian [21]. Previously, bovine and pigs have been the main sources of type I collagen. However, outbreaks of foot-and-mouth disease (FMD), transmissible spongiform encephalopathy (TSE) and bovine spongiform encephalopathy (BSE) over the past few decades have made it risky to use animal-derived type I collagen [22]. Several researchers have explored alternative sources of type I collagen. Studies have reported that marine organisms (invertebrates and crustose coralline algae) are good sources of type I collagen [22,23]. Large quantities of type I collagen can be extracted from marine organisms, and this collagen is metabolically compatible, requires fewer quality control and regulatory processing, few ethical and religious constraints compared to collagen harvested from terrestrial animals [24]. For instance, collagen extracted from rat-tail has high purity and the extraction process is simple [25]. Another approach used to minimize the potential risks of animal-derived type I collagen is the recombinant human collagen technology. However, the triple helix of recombinant human collagen is unstable. Type I collagen can also be extracted through acid treatment method [26]. The acid treatment method is used to extract type I collagen from caprine tendon, ovine tendon, porcine skin, bovine skin and marine fish. In the method, collagen is mainly extracted using acetic acid. The concentration of acetic acid reagent determines the solubility of the tissue and hence the yield of type I collagen [27]. In addition, the acid concentration alters electrostatic interactions, the structure and the final pH value of the tissue. However, acid extraction method is expensive, and the yield of type I collagen is low. The addition of enzymatic method can solve the problem of insufficient yield of type I collagen. Enzymes used to extract type I collagen include papain, trypsin, pepsin, metabolic enzymes and collagenase [28]. The enzymatic process comprises two phases. The first phase involves premixing the acid solution and enzymes to lyse the tissue by disrupting the interwoven collagen structure. The second phase involves collection of collagen with a low-concentration acidic solution. This two-step extraction method results in high yields of type I collagen. Factors such as extraction source, dialysis and pretreatment conditions determine the molecular weight, molecular structure and amino acid composition of the final collagen product [29].
Structural and biological characteristics of type I collagen
Type I collagen comprises four levels of structural organization. These include amino acid triplet, α-helix, triple helix and fibrils structures [11]. Type I collagen requires good mechanical strength and thermal stability to be effective as a scaffold. The triple helix structure contributes to the mechanical strength and thermal stability of type I collagen. In the subsequent section, details of the triple helix structure are explored and discussed. The triple helix of type I collagen was first reported by Pauling & Corey [30] and was named by Rich & Crick [31] and North [32]. The triple helix structure is 1.5 nm in diameter and approximately 300 nm in length. It comprises two identical α1(I)- and α1(II)-chains and a single α2(I)-chain comprising approximately 1000 amino acids [33]. Peptide models referred as collagen mimetic peptides (CMPs) or triple helical peptides (THPs) were developed to allow for easy and direct analysis of the triple helix. Using the models, the supercoiled triple helix was confirmed and the ligand-binding details were obtained, as well as sidechain interactions and hydrogen bonding patterns [34]. The three chains of the triple helix form a left-handed, rod-like helix structure. Each chain forms a common super spiral structure similar to the structure of polypropylene. Only glycine (Gly) can exist in the structure and forms a repeating (Gly-X-Y)n sequence owing to the space constraints caused by close packing of three chains. The positions X and Y are mainly occupied by proline (Pro) and hydroxyproline (Hyp) [35]. Hyp maintains thermal stability of type I collagen. Type I collagen has lower antigenicity and low immune rejection rates compared with other proteins owing to the simple and repeated (Gly-X-Y)n sequence. The triple helix structure allows combination with key binding proteins such as matrix-metalloproteases (MMPs), the von Will-ebrand factor and integrins [36]. Analysis of crystal structures shows the presence of hydrogen bonds on the amino group of Gly on one chain and on the carboxyl group of residues at position X on the adjacent chain. To explore the role of hydrogen bond formation, NMR hydrogen exchange studies were performed using 15N-labeled Gly-Leu-Ala residues located in the central region of peptide T3-785. It was found that the hydrogen bond enhances stability, and this effect is more important for regions lacking Pro residue. The complex interactions among amino acids, chemical bonds and other substances maintain structural stability of the triple helix. Further, the stability of the triple helix is crucial to the structural integrity of type I collagen. A stable triple helix enables type I collagen to withstand the body’s mechanical and physiological pressure, and allows it to adapt to the harmful stimulus caused by pH changes.
Type I collagen can be broken down by catabolic processes in tissues driven by collagenase. Collagenase enzymatic degradation of type I collagen is classified into external and internal degradation [37]. External degradation process involves binding of collagenase to the triple helix structure on the surface of type I collagen. After degradation of the triple helix structure, collagenase binds to the interior of type I collagen to execute internal degradation. Therefore, the degradation rate of type I collagen can be regulated using external cross-linkers.
Cross-linking of type I collagen
Scaffolds based on type I collagen lack enough mechanical strength and controllable degradation rate. Thus, cross-linkers are used to address these shortcomings. Structural properties of cartilage tissue and cross-linking strategies are discussed in the following section.
Effect of cross-linking in cartilage
Mammalian cartilage is a lymphatic connective tissue that lacks nerves, vessels and lymph. Cartilage is found in the spine, ribs, nose, external ears, airways and synovial joints [38,39] and has limited capacity for intrinsic healing or repair [40]. Three major types of cartilage have been identified in human beings, including fibrous cartilage, elastic cartilage and hyaline cartilage [41]. Hyaline cartilage is the most common cartilage type in human. It has a glassy appearance and is mainly found in articulating surfaces of ribs, noses, airways, synovial joints, trachea and growth plates. Fibrous cartilage type forms a transition between connective tissue and cartilage. Fresh fibrous cartilage is milky white, opaque and can stretch freely. Structurally, fibrous cartilage contains many collagen fiber bundles arranged in parallel or across the matrix. Fibrous cartilage is mainly distributed in the junction between the intervertebral disc, glenoid, articular cartilage disc, pubic symphysis, and the attachment part of the tendon and joint capsule ligament on articular cartilage. Moreover, fibrous cartilage contains more type I collagen than the other two types [42]. Elastic cartilage is mainly found in the auricle and epiglottis, and is highly elastic [43]. Because elastic cartilage contains more elastin fibers, the stretchability of elastic cartilage is better than that of the other two types of cartilage [44,45]. The fibers in the middle of the cartilage are denser compared with those at the edges. The three types of cartilage comprise chondrocytes which are involved in the synthesis and secretion of major components of ECM [46].
Load-bearing performance of articular cartilage is determined by the ability to maintain hydrated state during loading conditions [47,48]. Articular cartilage is generally divided into superficial, middle, deep layers and calcified zone. The superficial layer comprises flattened chondrocytes that are in contact with the synovial fluid and are responsible for the stretching function of the cartilage. The middle layer occupies 40-60% of the articular cartilage structure and mainly comprises proteoglycans and collagen. In addition, it contains spherical chondrocytes, which provide the first line of defense against stress. The deep layer contains collagen fibers with large diameters, enriched with glycosaminoglycans and the least amount of water. In the deep layer, there are columnar chondrocytes, which provide resistance to stress. The calcified layer anchors collagen fibers to the subchondral bone and the cartilage to the bone. Of note, the calcified layer contains less hypertrophic chondrocytes compared with the other layers (Figure 2). The middle layer is mainly composed of type II collagen, glycosaminogly and water, which interact with collagen fibrils and proteoglycan aggregates to provide load-bearing strength. Proteoglycan aggregates comprise a long hyaluronic acid backbone, containing high level of fixed negative charge at natural pH conditions. The repulsive effect of the negative charge enables proteoglycan aggregates to expand and draw large volumes of water into collagen fibrous network resulting in swelling pressure. The swelling pressure is alleviated by the binding effect of type II collagen fibrous grid [49]. Brown et al. [50] used the Benninghoff arcade fibril structure motif of cartilage to simulate the swelling response of cartilage. They developed an alternative model which was an improvement of the classical model that the deeper tissue near the calcified tissue zone provided some degree of strain-limiting properties. Further, the strong strain limiting properties are related to the orderly cross-linked type II collagen fibrous network, which cannot be achieved without an effectively cross-linked type II collagen fibrous network. The highly cross-linked collagen mesh structure maintains a “normal” configuration and provides greater resistance to extrusion and resilience relative to the loosely cross-linked mesh structure when a load is applied [51]. Resistance of cartilage to this deformation and volume change determines its ability to reshape and offer support. Research has shown that the mechanical strength and abrasion resistance of the cartilage are reduced when the articular cartilage is injured. However, when collagen cross-linker is applied, the abrasion resistance of the cartilage is repaired. Moreover, this enhances the resistance of the joint surface to collagen enzyme digestion. This illustrates the importance of collagen cross-linking to cartilage [52]. Moreover, during proliferation, chondrocytes can shrink, which leads to deformation of the scaffold thus inhibiting further cell growth. The shrinking scaffolds alter the overall condition of the structure and prevent integration of the implant into the host [53]. This shrinkage phenomenon has been explored through immunohistochemical analysis of α-smooth muscle actin (SMA) and contractile muscle protein present in human [54] and canine [55].
Figure 2.
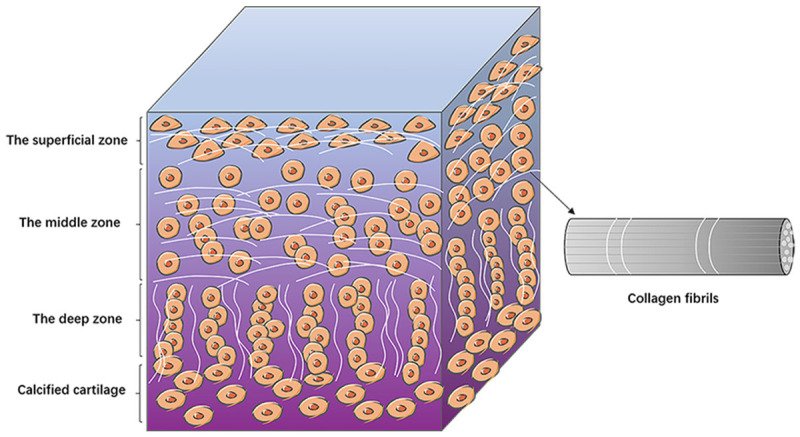
The structure of articular cartilage. The diagram shows the basic structures of the superficial, middle and deep layers of the articular cartilage. Flat chondrocytes are arranged in the superficial layer, oval chondrocytes in the middle layer, and strings of chondrocytes are vertically distributed in the deep layer. The collagen fibers are lined up with chondrocytes.
Effective cross-linking methods improve the mechanical strength of scaffolds and prevent cell-mediated contraction. Therefore, external cross-linking methods of type I collagen-based are used to optimize the mechanical properties of scaffolds in CTE. Reduction of degradation rate of scaffolds and stimulation of the collagen fibrous network inside cartilage allow effective development of scaffold-cell complexes. The main cross-linking methods are described in detail in the next section.
Cross-linking methods
Fabrication of scaffolds is crucial in the multidiscipline field of CTE. Due to its great biocompatibility and safety properties, type I collagen is a preferred biomaterial in CTE. As a result, numerous institutions such as pharmaceutical and medical device institutions, as well as food and drug regulation institutions, have approved type I collagen as a biomaterial source. However, pure type I collagen scaffolds have two limitations: poor mechanical properties [56] and difficulty in controlling the rate of biodegradation following implantation in the body. In practical applications, two methods are used to improve the mechanical strength of type I collagen scaffolds. These methods include: (1) a non-blending strategy that involves altering the physical form of the scaffolds, (2) a blending strategy that involves blending collagen with other biomaterials such as synthetic, natural and inorganic materials. These methods enhance the biological activity and mechanical strength of type I collagen scaffolds.
Biomaterials that can be combined with type I collagen in a hybrid method fall into three categories: (1) natural polymers, (2) synthetic polymers, and (3) inorganic materials. Natural polymer is a polymer complex generated in nature through biochemical action or photosynthesis in nature or by the action of minerals present in animals and plants. Examples of natural polymers include silk fibroin (SF), chitosan and cellulose. Blending type I collagen with synthetic polymers can increase the mechanical strength. Synthetic polymers such as poly ε-caprolactone (PCL), polylactic acid (PLA), poly ethylene glycol (PEG), polyglycolide (PGA), poly lactide-co-glycolide (PLGA) and polyvinyl alcohol (PVA), have been widely used in tissue engineering. The combination of type I collagen and inorganic materials in CTE has also been widely explored. Inorganic materials such as hydroxyapatite (HA, Ca10(PO4)6(OH)2), silicates and β-tricalcium phosphate (β-TCP, Ca3(PO4)2) are used to produce tissue engineering scaffolds.
Non-blending strategies are used to modify the physical properties of type I collagen scaffolds to increase their mechanical strength. Physical form modification entails transferring combinations of different matrix types without altering the chemical composition. The physical forms are divided into four types, including hydrogels, sponges, films and microspheres [57].
Notably, each of the aforementioned methods should be based on the cross-linking method. Cross-linking is the chemical or physical process through which polymer chains are joined together. It improves the mechanical properties of type I collagen scaffolds by forming a dense matrix network [58,59]. Cross-linking of type I collagen is achieved by stabilizing the side chains of amino acids side chains, which increases the stiffness of collagen fibers by inhibiting the long rod-like collagen molecules from sliding against one another under pressure [60]. Currently, the majority of type I collagen is obtained from animals [21], and extraction destroys the native cross-linking of type I collagen [61,62]. External methods should therefore be used to cross-link the extracted type I collagen. Therefore, choose of cross-linkers is very important. Cross-linkers are chosen based on a variety of factors, including their biological, physical and chemical properties and impact on the porosity of the scaffolds [63]. An ideal cross-linker should improve the mechanical strength of the scaffolds while remaining non-cytotoxic to cells [64]. Three methods of cross-linking methods have been described: physical, chemical and biological cross-linking methods (Table 1). The most often used technique is a chemical cross-linking method [65]. The next section discusses in depth the advantages and disadvantages of three cross-linking methods.
Table 1.
Classification of cross-linkers
| Type | Examples | Mechanism of action | Advantages | Disadvantages |
|---|---|---|---|---|
| Physical | 1. Dehydrothermal treatment (DHT) | Cross-linking by forming non-covalent bonds. | 1. Safe. | 1. Weak resistance to collagenase [68]. |
| 2. Ultraviolet irradiation (UV) | 2. Non-toxic to cells. | 2. Excessive temperature tends to cause collagen denaturation. | ||
| 3. Gentler action than chemical cross-linking. | 3. Poor crosslinking strength and durability. | |||
| 4. Inexpensive. | ||||
| 5. Extended biodegradation of scaffolds. | ||||
| Chemical | 1. Glutaraldehyde (GA) | Binds to chemical bond during cross-linking, leaves the chemical bond after cross-linking [97]. | 1. Virtually non-toxic to cells. | 1. Potential cytotoxicity to cells. |
| 2. Carbodiimide (EDC) | 2. Stronger cross-linking effect. | 2. Costly. | ||
| 3. Nhydroxysuccinimide (NHS) | 3. Strong resistance to collagenase. | 3. Require thorough washing to remove cross-linkers. | ||
| Biological | 1. Genipin | Cross-linking of two amide bonds formed by nucleophilic attack of the amine with two amino groups [89]. | 1. Natural sources. | 1. Excessive concentration can lead to a decrease in the mechanical strength of the scaffolds. |
| 2. Transglutaminase (TG) | 2. Good biocompatibility. | 2. Blue reaction after cross-linking. | ||
| 3. Plant-derived proanthocyanidins (PACs) | 3. Very low cytotoxicity. |
Physical cross-linking
Physical cross-linking method of type I collagen is mainly accomplished by the use of dehydrothermal (DHT) treatment and ultraviolet (UV) irradiation (Figure 3). UV irradiation crosslinks type I collagen by inducing physical and chemical changes in type I collagen. Cross-linking type I collagen using UV irradiation does not require further disinfection due to the sterilization effect of UV rays. However, if exposed to radiation over an extended period, type I collagen may be degraded. DHT treatment is a frequently used technique of cross-linking type I collagen. It is a physical treatment method that involves the removal of water from collagen at a high temperature under a vacuum, resulting in intermolecular cross-linking via condensation reactions caused by amide formation or esterification [66]. DHT treatment produces a mild cross-linking effect, which is similar to that of native cartilage [67]. Rowland et al. [67] examined the contraction of cartilage derived matrix scaffolds using various cross-linking procedures. According to the study, DHT treatment was often used in combination with UV as a base application for cross-linking. Additionally, when compared to the uncross-linked group, the DHT and UV cross-linked scaffolds retained their original volume and did not shrink when subjected to area testing. In a study by Kozłowska and Sionkowska [68], Col/Cap scaffolds cross-linked with DHT improved the compressive modulus from 130 kPa to 355 kPa, indicating that the DHT cross-linking enhanced the mechanical strength of the scaffolds. Apart from being non-toxic and easy to handle, UV has a powerful sterilizing effect on the scaffold. UV light at 254 nm sterilizes microorganisms by destroying their nucleic acids but has little effect on cell adhesion, spreading and proliferation of cells on type I collagen scaffolds [69]. Lee et al. [70] investigated the effect of cross-linking on the compressive stiffness of type I collagen-glycosaminoglycan scaffolds. The findings showed that the scaffolds cross-linked with DHT and UV treatment were resistant to the scaffold contraction caused by chondrocyte growth and proliferation; however, the effect was not obvious. DHT treatment and UV light crosslink type I collagen-based scaffolds without disrupting the scaffold-cell co-culture. Additionally, when DHT treatment is used, the degree of cross-linking increases with an increase in type I collagen concentration [71]. However, the application of such cross-linking methods alone is unable to meet the requirements for scaffold fabrication. Therefore, physical cross-linking is often used in combination with other cross-linking methods to maximize the effectiveness of type I collagen-based scaffolds.
Figure 3.
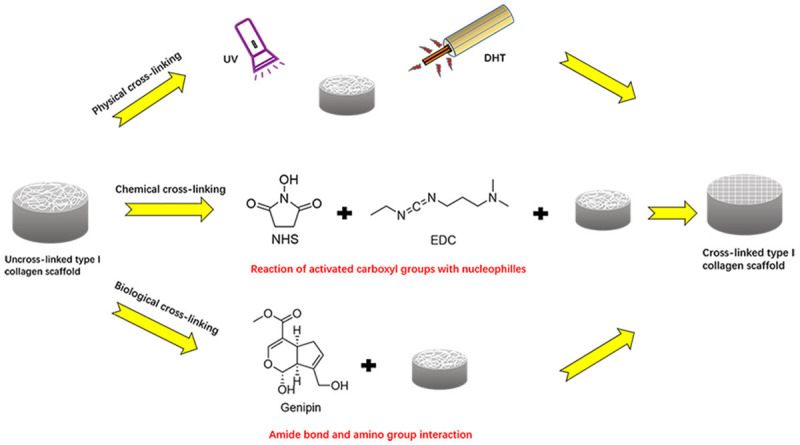
Different methods of cross-linking type I collagen scaffolds. Physical cross-linking includes UV irradiation and DHT heating treatments. Chemical cross-linking method is most commonly applied by combining EDC and NHS. In this combination, EDC activates aspartic acid and carboxyl groups of glutamic acid origin making them react with nucleophiles to form 0-length cross-links. Genipin induces cross-linking by initiating a nucleophilic attack on amine with two amino groups to form two amide bonds.
Chemical cross-linking
External cross-linking methods, mainly chemical cross-linking, are often used to reduce degradation rate and improve mechanical strength of type I collagen-based scaffolds. Chemical cross-linking method interconnects collagen fibrils to extend the ultrastructure duration and improve the mechanical strength of the scaffolds by reducing enzymatic degradation in vivo [72]. Commonly used chemical cross-linkers include glutaraldehyde (GTA), N-hydroxysuccinimide (NHS) and 1-ethyl-3-(3-dimethylaminopropyl) (EDC).
Chemical cross-linkers more effectively improve the mechanical strength of scaffolds than physical cross-linkers. Chemical cross-linking is the most suitable method for type I collagen-based scaffolds. This is because chemical cross-linkers improve the stability of scaffolds without causing structural changes. Unfortunately, most chemical cross-linkers are cytotoxic [73]. Therefore, the chemical cross-linkers must be cleaned thoroughly using deionized water before being used. In addition, the cost of chemical cross-linkers is more expensive than physical cross-linkers [64].
Evidence from previous studies shows that GTA is considered as an effective cross-linker for protein chemistry and tissue fixation that is less costly, with high activity and good solubility [74-76]. The reaction between type I collagen and GTA is mediated by interaction between the ε-amino group of the collagen molecule and the aldehyde group of GTA. Different amounts of GTA have different effects on the physicochemical properties of collagen solutions. When the ratio of GTA to collagen solution exceeds 0.1%, the thermal transition temperature of collagen solution suddenly increases and its fluidity decreases [77]. Notably, GTA has undesirable side effects such as induction of calcification, local cytotoxicity and induction of inflammatory response [78].
To be effective in cross-linking processes, EDC and NHS are usually combined [79]. EDC forms amide bonds with hydroxylysine and amines of lysine residues in the presence of NHS by activating the carboxyl groups of aspartic acid or glutamic acid residues, resulting in stable structures [80] (Figure 3). The two cross-linkers, EDC and NHS do not penetrate the cross-linked structure [81]. Furthermore, they are less toxic compared with GTA and can be safely used to crosslink collagen in tissues and scaffolds. Omobono [82] compared the resistance of type I collagen hydrogels to collagenase digestion after photo-crosslinking, EDC/NHS cross-linking and double cross-linking. They found that EDC/NHS cross-linkers induced toxic effects on chondrocytes. The findings showed that (1) Photochemical cross-linking increased resistance to collagenase digestion, however, it did not enhance the mechanical strength of type I collagen hydrogels; (2) EDC/NHS cross-linking resulted in stronger resistance to collagenase digestion and increased the strength of the scaffold, although it did not maintain the structural integrity of the scaffold; (3) Dual cross-linking (combination of photochemical and EDC/NHS) caused the strongest resistance, increased the mechanical strength of the scaffold and maintained structural integrity of the scaffold; (4) The three cross-linking methods had no toxic effects on chondrocytes. The effect of dual cross-linking was evaluated by measuring the storage modulus and stiffness of collagen gels. And the results showed that the effect of dual cross-linking was 5 times better than that of physical cross-linking alone. Han et al. [83] developed EDC/NHS cross-linked type I/II collagen composite scaffold and explored its biocompatibility with chondrocytes. The results showed that: (1) EDC/NHS cross-linking method enhanced the mechanical strength of scaffolds. Specially, the compressive strength of the cross-linked group was about 7 kPa higher than that of the uncross-linked group. The methods also increased the biological stability of the scaffolds and did not induce toxicity to chondrocytes. (2) The EDC/NHS cross-linking improved the proliferation-promoting and bioactivity-regulating effects of type I/II collagen composite scaffold on chondrocytes.
In summary, chemical cross-linkers such as GTA, NHS and EDC increase the mechanical strength of type I collagen-based scaffolds and are commonly used as cross-linkers for type I collagen. Both NHS and EDC have minimal toxic effects on chondrocytes and do not enter the cross-linked structure. Therefore, any residual minor toxic effects can be offset by thorough cleaning of the cross-linked scaffolds. However, EDC/NHS cross-linkers have low efficacy, therefore, they should be applied in combination with other cross-linking methods.
Biological cross-linking
Cross-linkers from biological sources have been developed in recent years to overcome the effects of physical cross-linkers and toxic effects of chemical cross-linking. Among the biological cross-linkers identified, genipin, transglutaminases, tyrosinase (Tyr), lysyl oxidase, phosphatases, horseradish peroxidase (HRP) and hydrogen peroxide (H2O2) have been developed [83]. Genipin has been widely used in recent years for cross-linking of type I collagen scaffolds for CTE due to its excellent cross-linking action and lack of toxicity to cells. In this section, the cross-linking effect of genipin on type I collagen-based scaffolds is mainly described.
Genipin is a hydrolysis product of genipin glycoside and is isolated from the fruits of Gardenia jasminoides Ellis [84]. Genipin reacts with the amino groups of proteins or amino acid to produce dark blue pigments [85,86]. Genipin has extremely low toxicity, and the toxic effect is reported to be 5000-10000 times lower compared with that of glutaraldehyde [87]. The use of genipin cross-linkers improves the mechanical strength and resistance to enzymatic degradation in type I collagen-based scaffolds [88]. The cross-linking effect of genipin is achieved through two phases: (1) formation of an aldehyde group at the C3 carbon atom of the original secondary amine source, and reaction of the secondary amine with the aldehyde group to form a heterocyclic compound; (2) substitution of the ester group on genipin by a secondary amide bond. Bi et al. [88] explored the effect of different concentrations of genipin on type I collagen/chitosan composite scaffolds. They found that genipin had two effects on the scaffolds: improved the mechanical strength of scaffolds and increased the internal pore size of type I collagen scaffolds (Figure 3). These two effects exist in a state of balance with each other. For instance, when the concentration of genipin is lower than 1.0%, it induced a higher mechanical strength than it increased the pore size. On the contrary, when the concentration of genipin is higher than 1.0%, it predominantly increases pore size while having little effect on the mechanical strength. These findings show that the cross-linking effect is maximum with a genipin concentration of 1.0%. Previous studies [89] reported that type I collagen/chitosan-based scaffolds cross-linked by genipin improved chondrocyte proliferation, and genipin increased stiffness of scaffolds from 9.53±2.2 kPa to 28.7±2.6 kPa. Moreover, cross-linking by genipin enhanced the resistance of cartilage to chemical degradation, reduced damage caused by mechanical wear and tear, and does not affect viability of chondrocytes [90,91]. A study by Zheng et al. [92] used type I collagen hydrogel as the main body of the scaffold to repair cartilage. Genipin was used to enhance the mechanical strength of the scaffold. Results obtained using SEM showed that the pore size of the scaffold was reduced from 100 μm to 50 μm after cross-linking with genipin, and this was accompanied by a blue reaction of genipin during cross-linking.
Other biological cross-linkers used in CTE include transglutaminase, which is an enzyme found in most organisms including vertebrates, plants and microorganisms. Transglutaminase plays a role in blood coagulation, regulation of red blood cell membranes and epidermal keratinization [93]. The advantages of using microbial transglutaminase (mTG) to cross-link cartilage scaffolds include low preparation cost and lack of dependence on calcium ions [94]. Furthermore, scaffolds crosslinked by mTG improve viability of chondrocytes cultured in vitro and do not induce inflammation [95-97]. Otherwise, other biological cross-linkers like ribose have also been confirmed to be a good cross-linker for type I collagen-based scaffolds [98].
Conclusions and future perspectives
In this review, we describe the current methods used to cross-link type I collagen scaffolds in CTE and the significance of cross-linking. Cartilage has low regeneration capacity. So far, CTE has been used to promote the repair of damaged cartilage. Successful implementation of CTE largely relies on availability of an effective scaffold. Type I collagen is widely used as a scaffold in CTE due to its good biocompatibility and easy to obtain from diverse sources. However, type I collagen-based scaffolds also have limitations such as rapid degradation rates and poor mechanical strength. Cross-linking can slow down these problems to some extent. Currently used cross-linking methods include physical cross-linking and chemical cross-linking. The latter is the most effective and widely used method, but its usage is associated with some degree of toxicity to cells. To overcome these shortcomings, the biological cross-linking approach has been developed in recent years. It should be noted that these cross-linking methods do not adequately meet the requirements of scaffold fabrication when applied alone. To address this, superimposed applications have been developed, which also increase the operational difficulty, cost, and possible harm to cells. Therefore, in the subsequent cartilage tissue engineering research, there is a need to develop a cross-linker for cross-linking type I collagen scaffolds that is almost non-toxic to cells, can be applied alone, has low fabrication cost, widely available, and is simple to use. In the future, cross-linkers of biological origin deserve further attention due to their safety and non-toxic nature.
Acknowledgements
We acknowledge all authors who contributed to this work. Supported by the Chinese Academy of Medical Sciences (CAMS) grant: CAMS-2017-I2M-1-007.
Disclosure of conflict of interest
None.
References
- 1.Goel SC. Cartilage regeneration. Indian Journal of Orthopaedics. 2006;40:303. [Google Scholar]
- 2.Qasim M, Chae DS, Lee NY. Advancements and frontiers in nano-based 3D and 4D scaffolds for bone and cartilage tissue engineering. Int J Nanomedicine. 2019;14:4333–4351. doi: 10.2147/IJN.S209431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glowacki J, Mizuno S. Collagen scaffolds for tissue engineering. Biopolymers. 2008;89:338–344. doi: 10.1002/bip.20871. [DOI] [PubMed] [Google Scholar]
- 4.Levinson C, Cavalli E, von Rechenberg B, Zenobi-Wong M, Darwiche SE. Combination of a collagen scaffold and an adhesive hyaluronan-based hydrogel for cartilage regeneration: a proof of concept in an ovine model. Cartilage. 2021;13(Suppl):636S–649S. doi: 10.1177/1947603521989417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daly AC, Freeman FE, Gonzalez-Fernandez T, Critchley SE, Nulty J, Kelly DJ. 3D bioprinting for cartilage and osteochondral tissue engineering. Adv Healthc Mater. 2017;6 doi: 10.1002/adhm.201700298. [DOI] [PubMed] [Google Scholar]
- 6.Wang CC, Yang KC, Lin KH, Liu HC, Lin FH. A highly organized three-dimensional alginate scaffold for cartilage tissue engineering prepared by microfluidic technology. Biomaterials. 2011;32:7118–7126. doi: 10.1016/j.biomaterials.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Huang K, Li Q, Li Y, Yao Z, Luo D, Rao P, Xiao J. Cartilage tissue regeneration: the roles of cells, stimulating factors and scaffolds. Curr Stem Cell Res Ther. 2018;13:547–567. doi: 10.2174/1574888X12666170608080722. [DOI] [PubMed] [Google Scholar]
- 8.Kim BS, Mooney DJ. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998;16:224–230. doi: 10.1016/s0167-7799(98)01191-3. [DOI] [PubMed] [Google Scholar]
- 9.Kwon H, Rainbow RS, Sun L, Hui CK, Cairns DM, Preda RC, Kaplan DL, Zeng L. Scaffold structure and fabrication method affect proinflammatory milieu in three-dimensional-cultured chondrocytes. J Biomed Mater Res A. 2015;103:534–544. doi: 10.1002/jbm.a.35203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi D, Xu X, Ye Y, Song K, Cheng Y, Di J, Hu Q, Li J, Ju H, Jiang Q, Gu Z. Photo-crosslinked scaffold with kartogenin-encapsulated nanoparticles for cartilage regeneration. ACS Nano. 2016;10:1292–1299. doi: 10.1021/acsnano.5b06663. [DOI] [PubMed] [Google Scholar]
- 11.Gelse K, Pöschl E, Aigner T. Collagens--structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Bartolomeo R, Cinque L, De Leonibus C, Forrester A, Salzano AC, Monfregola J, De Gennaro E, Nusco E, Azario I, Lanzara C, Serafini M, Levine B, Ballabio A, Settembre C. mTORC1 hyperactivation arrests bone growth in lysosomal storage disorders by suppressing autophagy. J Clin Invest. 2017;127:3717–3729. doi: 10.1172/JCI94130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burgeson RE, Nimni ME. Collagen types. Molecular structure and tissue distribution. Clin Orthop Relat Res. 1992:250–272. [PubMed] [Google Scholar]
- 14.Miyata T, Taira T, Noishiki Y. Collagen engineering for biomaterial use. Clin Mater. 1992;9:139–148. doi: 10.1016/0267-6605(92)90093-9. [DOI] [PubMed] [Google Scholar]
- 15.Burla F, Dussi S, Martinez-Torres C, Tauber J, van der Gucht J, Koenderink GH. Connectivity and plasticity determine collagen network fracture. Proc Natl Acad Sci U S A. 2020;117:8326–8334. doi: 10.1073/pnas.1920062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller EJ, Gay S. Collagen: an overview. Methods Enzymol. 1982;82:3–32. doi: 10.1016/0076-6879(82)82058-2. [DOI] [PubMed] [Google Scholar]
- 17.Brinckmann J. Collagens at a Glance. Top Curr Chem. 2005;247:1–6. [Google Scholar]
- 18.Miki A, Inaba S, Baba T, Kihira K, Fukada H, Oda M. Structural and physical properties of collagen extracted from moon jellyfish under neutral pH conditions. Biosci Biotechnol Biochem. 2015;79:1603–1607. doi: 10.1080/09168451.2015.1046367. [DOI] [PubMed] [Google Scholar]
- 19.Salvatore L, Gallo N, Natali ML, Terzi A, Sannino A, Madaghiele M. Mimicking the hierarchical organization of natural collagen: toward the development of ideal scaffolding material for tissue regeneration. Front Bioeng Biotechnol. 2021;9:644595. doi: 10.3389/fbioe.2021.644595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shekhter AB, Fayzullin AL, Vukolova MN, Rudenko TG, Osipycheva VD, Litvitsky PF. Medical applications of collagen and collagen-based materials. Curr Med Chem. 2019;26:506–516. doi: 10.2174/0929867325666171205170339. [DOI] [PubMed] [Google Scholar]
- 21.McKee RA, Wingert RA. Repopulating decellularized kidney scaffolds: an avenue for ex vivo organ generation. Materials (Basel) 2016;9:190. doi: 10.3390/ma9030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felician FF, Xia C, Qi W, Xu H. Collagen from marine biological sources and medical applications. Chem Biodivers. 2018;15:e1700557. doi: 10.1002/cbdv.201700557. [DOI] [PubMed] [Google Scholar]
- 23.Orgel J, Sella I, Madhurapantula RS, Antipova O, Mandelberg Y, Kashman Y, Benayahu D, Benayahu Y. Molecular and ultrastructural studies of a fibrillar collagen from octocoral (Cnidaria) J Exp Biol. 2017;220:3327–3335. doi: 10.1242/jeb.163824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahman MA. Collagen of extracellular matrix from marine invertebrates and its medical applications. Mar Drugs. 2019;17:118. doi: 10.3390/md17020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Netzlaff F, Lehr CM, Wertz PW, Schaefer UF. The human epidermis models EpiSkin, SkinEthic and EpiDerm: an evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur J Pharm Biopharm. 2005;60:167–178. doi: 10.1016/j.ejpb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Zou Y, Wang L, Cai P, Li P, Zhang M, Sun Z, Sun C, Xu W, Wang D. Effect of ultrasound assisted extraction on the physicochemical and functional properties of collagen from soft-shelled turtle calipash. Int J Biol Macromol. 2017;105:1602–1610. doi: 10.1016/j.ijbiomac.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Liu D, Wei G, Li T, Hu J, Lu N, Regenstein JM, Zhou P. Effects of alkaline pretreatments and acid extraction conditions on the acid-soluble collagen from grass carp (Ctenopharyngodon idella) skin. Food Chem. 2015;172:836–843. doi: 10.1016/j.foodchem.2014.09.147. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt MM, Dornelles R, Mello RO, Kubota EH, Demiate IM. Collagen extraction process. International Food Research Journal. 2016 [Google Scholar]
- 29.Skopinska-Wisniewska J, Olszewski K, Bajek A, Rynkiewicz A, Sionkowska A. Dialysis as a method of obtaining neutral collagen gels. Mater Sci Eng C Mater Biol Appl. 2014;40:65–70. doi: 10.1016/j.msec.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 30.Pauling L, Corey RB. The structure of fibrous proteins of the collagen-gelatin group. Proc Natl Acad Sci U S A. 1951;37:272–281. doi: 10.1073/pnas.37.5.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller A, Bradshaw J, Jones EY, Fraser RD, Macrae TP, Suzuki E. The Structure of Collagen. Ciba Found Symp. 1985;114:65–79. doi: 10.1002/9780470720950.ch5. [DOI] [PubMed] [Google Scholar]
- 32.Cowan PM, McGavin S, North AC. The polypeptide chain configuration of collagen. Nature. 1955;176:1062–1064. doi: 10.1038/1761062a0. [DOI] [PubMed] [Google Scholar]
- 33.Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem J. 1996;316:1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brodsky B, Persikov AV. Molecular structure of the collagen triple helix. Adv Protein Chem. 2005;70:301–339. doi: 10.1016/S0065-3233(05)70009-7. [DOI] [PubMed] [Google Scholar]
- 35.Ramshaw JA, Shah NK, Brodsky B. Gly-X-Y tripeptide frequencies in collagen: a context for host-guest triple-helical peptides. J Struct Biol. 1998;122:86–91. doi: 10.1006/jsbi.1998.3977. [DOI] [PubMed] [Google Scholar]
- 36.Stultz CM. Localized unfolding of collagen explains collagenase cleavage near imino-poor sites. J Mol Biol. 2002;319:997–1003. doi: 10.1016/S0022-2836(02)00421-7. [DOI] [PubMed] [Google Scholar]
- 37.Dong C, Lv Y. Application of collagen scaffold in tissue engineering: recent advances and new perspectives. Polymers (Basel) 2016;8:42. doi: 10.3390/polym8020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porter JR, Ruckh TT, Popat KC. Bone tissue engineering: a review in bone biomimetics and drug delivery strategies. Biotechnol Prog. 2009;25:1539–1560. doi: 10.1002/btpr.246. [DOI] [PubMed] [Google Scholar]
- 39.Krishnan Y, Grodzinsky AJ. Cartilage diseases. Matrix Biol. 2018;71-72:51–69. doi: 10.1016/j.matbio.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1:461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wachsmuth L, Söder S, Fan Z, Finger F, Aigner T. Immunolocalization of matrix proteins in different human cartilage subtypes. Histol Histopathol. 2006;21:477–485. doi: 10.14670/HH-21.477. [DOI] [PubMed] [Google Scholar]
- 42.Bielajew BJ, Hu JC, Athanasiou KA. Methodology to quantify collagen subtypes and crosslinks: application in minipig cartilages. Cartilage. 2021;13(Suppl):1742S–1754S. doi: 10.1177/19476035211060508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sturm A, Chaiet SR. Chondrolaryngoplasty-thyroid cartilage reduction. Facial Plast Surg Clin North Am. 2019;27:267–272. doi: 10.1016/j.fsc.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Bielajew BJ, Hu JC, Athanasiou KA. Collagen: quantification, biomechanics, and role of minor subtypes in cartilage. Nat Rev Mater. 2020;5:730–747. doi: 10.1038/s41578-020-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Visscher DO, Lee H, van Zuijlen PPM, Helder MN, Atala A, Yoo JJ, Lee SJ. A photo-crosslinkable cartilage-derived extracellular matrix bioink for auricular cartilage tissue engineering. Acta Biomater. 2021;121:193–203. doi: 10.1016/j.actbio.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. The extracellular matrix: tools and insights for the “omics” era. Matrix Biol. 2016;49:10–24. doi: 10.1016/j.matbio.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maroudas A, Bannon C. Measurement of swelling pressure in cartilage and comparison with the osmotic pressure of constituent proteoglycans. Biorheology. 1981;18:619–632. doi: 10.3233/bir-1981-183-624. [DOI] [PubMed] [Google Scholar]
- 48.Horkay F, Basser PJ. Composite hydrogel model of cartilage predicts its load-bearing ability. Sci Rep. 2020;10:8103. doi: 10.1038/s41598-020-64917-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Broom ND, Marra DL. New structural concepts of articular cartilage demonstrated with a physical model. Connect Tissue Res. 1985;14:1–8. doi: 10.3109/03008208509089838. [DOI] [PubMed] [Google Scholar]
- 50.Brown ETT, Damen AHA, Thambyah A. The mechanical significance of the zonally differentiated collagen network of articular cartilage in relation to tissue swelling. Clin Biomech (Bristol, Avon) 2020;79:104926. doi: 10.1016/j.clinbiomech.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Chen YC, Chen M, Gaffney EA, Brown CP. Effect of crosslinking in cartilage-like collagen microstructures. J Mech Behav Biomed Mater. 2017;66:138–143. doi: 10.1016/j.jmbbm.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Bonitsky CM, McGann ME, Selep MJ, Ovaert TC, Trippel SB, Wagner DR. Genipin crosslinking decreases the mechanical wear and biochemical degradation of impacted cartilage in vitro. J Orthop Res. 2017;35:558–565. doi: 10.1002/jor.23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng NC, Estes BT, Young TH, Guilak F. Genipin-crosslinked cartilage-derived matrix as a scaffold for human adipose-derived stem cell chondrogenesis. Tissue Eng Part A. 2013;19:484–496. doi: 10.1089/ten.tea.2012.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim AC, Spector M. Distribution of chondrocytes containing alpha-smooth muscle actin in human articular cartilage. J Orthop Res. 2000;18:749–755. doi: 10.1002/jor.1100180511. [DOI] [PubMed] [Google Scholar]
- 55.Wang Q, Breinan HA, Hsu HP, Spector M. Healing of defects in canine articular cartilage: distribution of nonvascular alpha-smooth muscle actin-containing cells. Wound Repair Regen. 2000;8:145–158. doi: 10.1046/j.1524-475x.2000.00145.x. [DOI] [PubMed] [Google Scholar]
- 56.Higuchi A, Ling QD, Hsu ST, Umezawa A. Biomimetic cell culture proteins as extracellular matrices for stem cell differentiation. Chem Rev. 2012;112:4507–4540. doi: 10.1021/cr3000169. [DOI] [PubMed] [Google Scholar]
- 57.Kim MH, Kino-Oka M. A novel strategy for simple and robust expansion of human pluripotent stem cells using botulinum hemagglutinin. Adv Exp Med Biol. 2018;1077:19–29. doi: 10.1007/978-981-13-0947-2_2. [DOI] [PubMed] [Google Scholar]
- 58.Daemi H, Barikani M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Scientia Iranica. 2012;19:2023–2028. [Google Scholar]
- 59.Oryan A, Kamali A, Moshiri A, Baharvand H, Daemi H. Chemical crosslinking of biopolymeric scaffolds: current knowledge and future directions of crosslinked engineered bone scaffolds. Int J Biol Macromol. 2018;107:678–688. doi: 10.1016/j.ijbiomac.2017.08.184. [DOI] [PubMed] [Google Scholar]
- 60.Bailey AJ, Light ND, Atkins ED. Chemical cross-linking restrictions on models for the molecular organization of the collagen fibre. Nature. 1980;288:408–410. doi: 10.1038/288408a0. [DOI] [PubMed] [Google Scholar]
- 61.Cliche S, Amiot J, Avezard C, Gariépy C. Extraction and characterization of collagen with or without telopeptides from chicken skin. Poult Sci. 2003;82:503–509. doi: 10.1093/ps/82.3.503. [DOI] [PubMed] [Google Scholar]
- 62.Friess W. Collagen--biomaterial for drug delivery. Eur J Pharm Biopharm. 1998;45:113–136. doi: 10.1016/s0939-6411(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 63.Reddy N, Li Y, Yang Y. Alkali-catalyzed low temperature wet crosslinking of plant proteins using carboxylic acids. Biotechnol Prog. 2009;25:139–146. doi: 10.1002/btpr.86. [DOI] [PubMed] [Google Scholar]
- 64.Esfandiari F, Ashtiani MK, Sharifi-Tabar M, Saber M, Daemi H, Ghanian MH, Shahverdi A, Baharvand H. Microparticle-mediated delivery of BMP4 for generation of meiosis-competent germ cells from embryonic stem cells. Macromol Biosci. 2017;17 doi: 10.1002/mabi.201600284. [DOI] [PubMed] [Google Scholar]
- 65.Tang X, Bruce JE. Chemical cross-linking for protein-protein interaction studies. Methods Mol Biol. 2009;492:283–293. doi: 10.1007/978-1-59745-493-3_17. [DOI] [PubMed] [Google Scholar]
- 66.Yannas IV, Tobolsky AV. Cross-linking of gelatine by dehydration. Nature. 1967;215:509–510. doi: 10.1038/215509b0. [DOI] [PubMed] [Google Scholar]
- 67.Rowland CR, Lennon DP, Caplan AI, Guilak F. The effects of crosslinking of scaffolds engineered from cartilage ECM on the chondrogenic differentiation of MSCs. Biomaterials. 2013;34:5802–5812. doi: 10.1016/j.biomaterials.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kozłowska J, Sionkowska A. Effects of different crosslinking methods on the properties of collagen-calcium phosphate composite materials. Int J Biol Macromol. 2015;74:397–403. doi: 10.1016/j.ijbiomac.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 69.Davidenko N, Bax DV, Schuster CF, Farndale RW, Hamaia SW, Best SM, Cameron RE. Optimisation of UV irradiation as a binding site conserving method for crosslinking collagen-based scaffolds. J Mater Sci Mater Med. 2016;27:14. doi: 10.1007/s10856-015-5627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee CR, Grodzinsky AJ, Spector M. The effects of cross-linking of collagen-glycosaminoglycan scaffolds on compressive stiffness, chondrocyte-mediated contraction, proliferation and biosynthesis. Biomaterials. 2001;22:3145–3154. doi: 10.1016/s0142-9612(01)00067-9. [DOI] [PubMed] [Google Scholar]
- 71.Suesca E, Dias AMA, Braga MEM, de Sousa HC, Fontanilla MR. Multifactor analysis on the effect of collagen concentration, cross-linking and fiber/pore orientation on chemical, microstructural, mechanical and biological properties of collagen type I scaffolds. Mater Sci Eng C Mater Biol Appl. 2017;77:333–341. doi: 10.1016/j.msec.2017.03.243. [DOI] [PubMed] [Google Scholar]
- 72.Reddy N, Reddy R, Jiang Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015;33:362–369. doi: 10.1016/j.tibtech.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 73.Zhang J, Tang Q, Zhou A, Yang S. [Research progress of collagen-based three-dimensional porous scaffolds used in skin tissue engineering] . Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2015;32:924–928. [PubMed] [Google Scholar]
- 74.Migneault I, Dartiguenave C, Bertrand MJ, Waldron KC. Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques. 2004;37:790–796. 798–802. doi: 10.2144/04375RV01. [DOI] [PubMed] [Google Scholar]
- 75.Cheung DT, Tong D, Perelman N, Ertl D, Nimni ME. Mechanism of crosslinking of proteins by glutaraldehyde. IV: In vitro and in vivo stability of a crosslinked collagen matrix. Connect Tissue Res. 1990;25:27–34. doi: 10.3109/03008209009009810. [DOI] [PubMed] [Google Scholar]
- 76.Hansen P, Hassenkam T, Svensson RB, Aagaard P, Trappe T, Haraldsson BT, Kjaer M, Magnusson P. Glutaraldehyde cross-linking of tendon--mechanical effects at the level of the tendon fascicle and fibril. Connect Tissue Res. 2009;50:211–222. doi: 10.1080/03008200802610040. [DOI] [PubMed] [Google Scholar]
- 77.Tian Z, Li C, Duan L, Li G. Physicochemical properties of collagen solutions cross-linked by glutaraldehyde. Connect Tissue Res. 2014;55:239–247. doi: 10.3109/03008207.2014.898066. [DOI] [PubMed] [Google Scholar]
- 78.Lee JM, Edwards H, Pereira CA, Samii SI. Crosslinking of tissue-derived biomaterials in 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) Journal of Materials Science Materials in Medicine. 1996;7:531–541. [Google Scholar]
- 79.Nong LM, Zhou D, Zheng D, Jiang YQ, Xu NW, Zhao GY, Wei H, Zhou SY, Han H, Han L. The effect of different cross-linking conditions of EDC/NHS on type II collagen scaffolds: an in vitro evaluation. Cell Tissue Bank. 2019;20:557–568. doi: 10.1007/s10561-019-09790-7. [DOI] [PubMed] [Google Scholar]
- 80.Angele P, Abke J, Kujat R, Faltermeier H, Schumann D, Nerlich M, Kinner B, Englert C, Ruszczak Z, Mehrl R, Mueller R. Influence of different collagen species on physico-chemical properties of crosslinked collagen matrices. Biomaterials. 2004;25:2831–2841. doi: 10.1016/j.biomaterials.2003.09.066. [DOI] [PubMed] [Google Scholar]
- 81.Ma DH, Lai JY, Cheng HY, Tsai CC, Yeh LK. Carbodiimide cross-linked amniotic membranes for cultivation of limbal epithelial cells. Biomaterials. 2010;31:6647–6658. doi: 10.1016/j.biomaterials.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 82.Omobono MA, Zhao X, Furlong MA, Kwon CH, Gill TJ, Randolph MA, Redmond RW. Enhancing the stiffness of collagen hydrogels for delivery of encapsulated chondrocytes to articular lesions for cartilage regeneration. J Biomed Mater Res A. 2015;103:1332–1338. doi: 10.1002/jbm.a.35266. [DOI] [PubMed] [Google Scholar]
- 83.Han L, Zhang ZW, Wang BH, Wen ZK. Construction and biocompatibility of a thin type I/II collagen composite scaffold. Cell Tissue Bank. 2018;19:47–59. doi: 10.1007/s10561-017-9653-2. [DOI] [PubMed] [Google Scholar]
- 84.Sung HW, Chang Y, Liang IL, Chang WH, Chen YC. Fixation of biological tissues with a naturally occurring crosslinking agent: fixation rate and effects of pH, temperature, and initial fixative concentration. J Biomed Mater Res. 2000;52:77–87. doi: 10.1002/1097-4636(200010)52:1<77::aid-jbm10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 85.Cauich-Rodriguez JV, Deb S, Smith R. Effect of cross-linking agents on the dynamic mechanical properties of hydrogel blends of poly(acrylic acid)-poly(vinyl alcohol-vinyl acetate) Biomaterials. 1996;17:2259–2264. doi: 10.1016/0142-9612(96)00058-0. [DOI] [PubMed] [Google Scholar]
- 86.Wang Z, Liu H, Luo W, Cai T, Li Z, Liu Y, Gao W, Wan Q, Wang X, Wang J, Wang Y, Yang X. Regeneration of skeletal system with genipin crosslinked biomaterials. J Tissue Eng. 2020;11:2041731420974861. doi: 10.1177/2041731420974861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sung HW, Huang RN, Huang LL, Tsai CC, Chiu CT. Feasibility study of a natural crosslinking reagent for biological tissue fixation. J Biomed Mater Res. 1998;42:560–567. doi: 10.1002/(sici)1097-4636(19981215)42:4<560::aid-jbm12>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 88.Bi L, Cao Z, Hu Y, Song Y, Yu L, Yang B, Mu J, Huang Z, Han Y. Effects of different cross-linking conditions on the properties of genipin-cross-linked chitosan/collagen scaffolds for cartilage tissue engineering. J Mater Sci Mater Med. 2011;22:51–62. doi: 10.1007/s10856-010-4177-3. [DOI] [PubMed] [Google Scholar]
- 89.Wang PY, Tsai WB. Modulation of the proliferation and matrix synthesis of chondrocytes by dynamic compression on genipin-crosslinked chitosan/collagen scaffolds. J Biomater Sci Polym Ed. 2013;24:507–519. doi: 10.1080/09205063.2012.696310. [DOI] [PubMed] [Google Scholar]
- 90.McGann ME, Bonitsky CM, Jackson ML, Ovaert TC, Trippel SB, Wagner DR. Genipin crosslinking of cartilage enhances resistance to biochemical degradation and mechanical wear. J Orthop Res. 2015;33:1571–1579. doi: 10.1002/jor.22939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bonitsky CM, McGann ME, Selep MJ, Ovaert TC, Trippel SB, Wagner DR. Genipin crosslinking decreases the mechanical wear and biochemical degradation of impacted cartilage in vitro. J Orthop Res. 2017;35:558–565. doi: 10.1002/jor.23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zheng L, Liu S, Cheng X, Qin Z, Lu Z, Zhang K, Zhao J. Intensified stiffness and photodynamic provocation in a collagen-based composite hydrogel drive chondrogenesis. Adv Sci (Weinh) 2019;6:1900099. doi: 10.1002/advs.201900099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Masahiko , Nonaka H, Tanaka A, Okiyama M, Motoki H, Ando K, Umeda A, Agricultural MJ, Chemistry B. Polymerization of several proteins by Ca2+-independent transglutaminase derived from microorganisms. 1989 [Google Scholar]
- 94.Sommer C, Hertel TC, Schmelzer CE, Pietzsch M. Investigations on the activation of recombinant microbial pro-transglutaminase: in contrast to proteinase K, dispase removes the histidine-tag. Amino Acids. 2012;42:997–1006. doi: 10.1007/s00726-011-1016-x. [DOI] [PubMed] [Google Scholar]
- 95.Schwarz S, Kuth S, Distler T, Gögele C, Stölzel K, Detsch R, Boccaccini AR, Schulze-Tanzil G. 3D printing and characterization of human nasoseptal chondrocytes laden dual crosslinked oxidized alginate-gelatin hydrogels for cartilage repair approaches. Mater Sci Eng C Mater Biol Appl. 2020;116:111189. doi: 10.1016/j.msec.2020.111189. [DOI] [PubMed] [Google Scholar]
- 96.Tsai CC, Kuo SH, Lu TY, Cheng NC, Shie MY, Yu J. Enzyme-cross-linked gelatin hydrogel enriched with an articular cartilage extracellular matrix and human adipose-derived stem cells for hyaline cartilage regeneration of rabbits. ACS Biomater Sci Eng. 2020;6:5110–5119. doi: 10.1021/acsbiomaterials.9b01756. [DOI] [PubMed] [Google Scholar]
- 97.Olde Damink LH, Dijkstra PJ, van Luyn MJ, van Wachem PB, Nieuwenhuis P, Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials. 1996;17:765–773. doi: 10.1016/0142-9612(96)81413-x. [DOI] [PubMed] [Google Scholar]
- 98.Gostynska N, Shankar Krishnakumar G, Campodoni E, Panseri S, Montesi M, Sprio S, Kon E, Marcacci M, Tampieri A, Sandri M. 3D porous collagen scaffolds reinforced by glycation with ribose for tissue engineering application. Biomed Mater. 2017;12:055002. doi: 10.1088/1748-605X/aa7694. [DOI] [PubMed] [Google Scholar]


