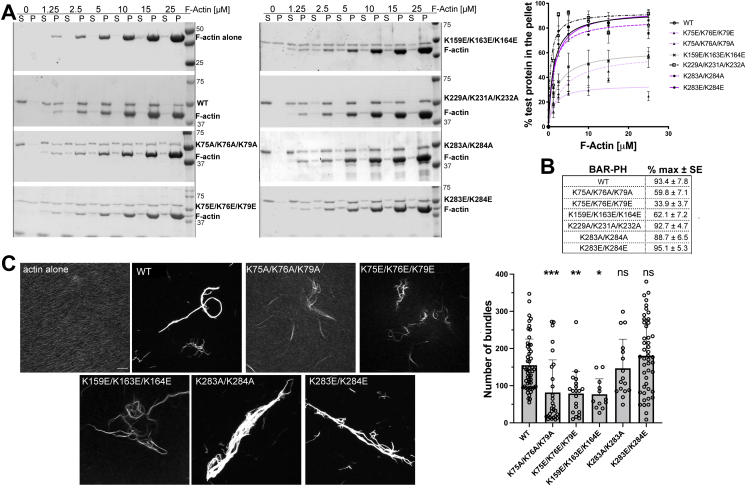
Figure 2.
Basic patch involving amino acids 75, 76, and 79 on ASAP1 BAR-PH is critical for binding and bundling F-actin.A and B, results of high-speed actin cosedimentation experiments show control actin filament sedimentation (F-actin alone), cosedimentation of actin filaments with WT ASAP1 BAR-PH (WT), [K229A, K231A, K232A], [K283A, K284A], and [K283E, K284E] mutants. Cosedimentation of ASAP1 [K75A, K76A, K79A], [K75E, K76E, K79E], and [K159E, K163E, K164E] with actin filaments is impaired. Quantification of actin filament binding by ASAP1 BAR-PH mutants and maximal binding are presented in the graph (A, % test protein in the pellet, mean ± SEM) and in tabular form (B, % max and SE), respectively. N = 2 to 3 independent experiments. C, representative images of fluorescence-based actin bundling assay in the absence or presence of ASAP1 BAR-PH, and mutants and graph summarizing quantification of the number of bundles (mean ± SD) induced by each protein. F-actin was incubated alone or with BAR-PH on glass coverslips and the bundles were stained with fluorescent phalloidin and assessed using confocal microscopy. The scale bar represents 10 μm, N = 3 independent experiments with 10 to 15 replicates. BAR, Bin/Amphiphysin/Rvs domain; F-actin, filamentous actin; ns, not significant; P, pellet; PH, pleckstrin homology; S, supernatant. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.