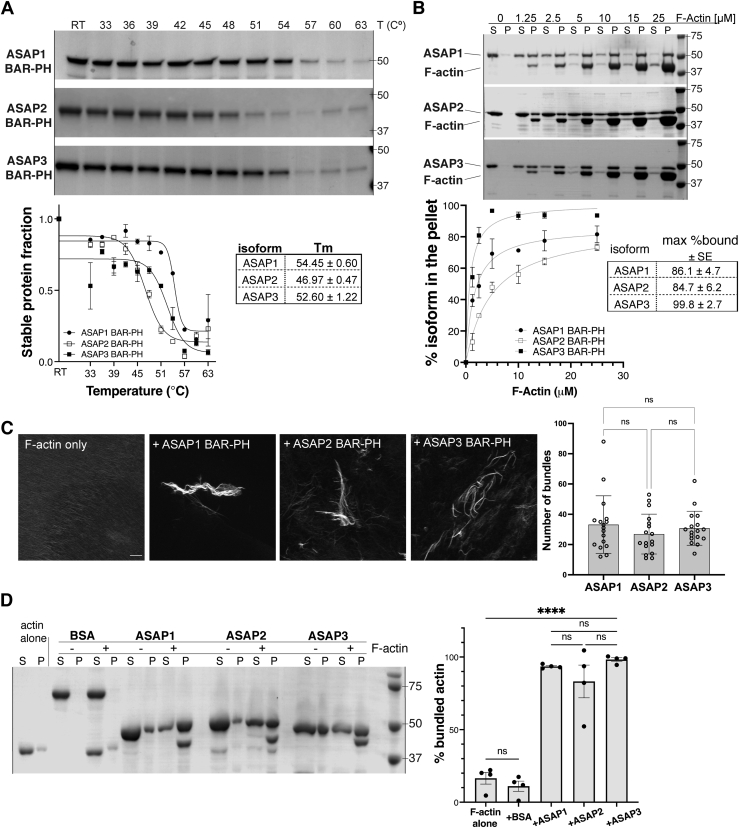
Figure 6.
In vitro actin filament binding and bundling activity of BAR-PH region is conserved in ASAP subtypes.A, thermal stability of purified BAR-PH of ASAP1 — three was assessed as in Fig. S1. B, results of high-speed cosedimentation assay show the sedimentation of actin filaments with BAR-PH of ASAP1, ASAP2, and ASAP3, with a graph summarizing the quantification of actin binding (% protein in the pellet, mean ± SEM) and a table presenting approximate binding maxima. N = 2. C, results of fluorescence-based actin bundling assay in the absence or presence of ASAP1, ASAP2, or ASAP3. The images are representative of two independent experiments. The scale bar represents 10 μm. The graph summarizes quantification of the number of bundles produced by the BAR-PH regions of ASAP1, 2, or 3 (mean ± SD). D, results of actin low-speed cosedimentation-based bundling assays in the absence or presence of BSA (negative control), ASAP1, ASAP2, or ASAP3 BAR-PH. F-actin was sedimented alone (actin alone) at low speed as a control for aggregation. Bovine serum albumin and ASAPs were sedimented alone or in the presence of actin filaments, and bundle formation (% bundled actin) was quantified using densitometric analysis. The graph summarizes the results from four independent experiments, mean ± SD. n.s. – not significant, ∗∗∗∗p < 0.0001 using One-way ANOVA with Dunnet’s multiple comparison test. BAR, Bin/Amphiphysin/Rvs domain; BSA, bovine serum albumin; F-actin, filamentous actin; PH, pleckstrin homology.