Abstract
Context
The number of reported cases with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) vaccine–induced subacute thyroiditis (SAT) and Graves’ disease (GD) is growing. However, active debate continues about managing such side effects and the safety of repeat or booster doses of the vaccines in such cases.
Objectives
This study aims to present long-term clinical follow-up of SARS-CoV-2 vaccine–induced SAT or GD cases and provide data regarding the safety of revaccinations.
Methods
Patients diagnosed with SARS-CoV-2 vaccine–induced SAT or GD were included. Data regarding the long-term clinical follow-up of SARS-CoV-2 vaccine–induced SAT and GD cases and outcomes of repeat or booster SARS-CoV-2 vaccinations were documented. The literature, including cases of SARS-CoV-2 vaccine–induced SAT or GD, was reviewed.
Results
Fifteen patients with SARS-CoV-2 vaccine–induced SAT and 4 with GD were included. Pfizer/BioNTech COVID-19 vaccine (BNT162b2) was associated with symptoms in a majority of cases with SAT and all with GD. Median time from vaccination to symptom onset was 7 and 11.5 days, respectively, while 7 and 2 patients required medical treatment in SAT and GD groups, respectively. Remission was documented in 10 SAT patients, with a median time to remission of 11.5 weeks. No exacerbation/recurrence of SAT occurred in 7 of 9 patients who received a repeat vaccination dose, while symptoms of SAT worsened following the second vaccination in 2 cases. None of the patients experienced severe side effects that could be associated with revaccinations.
Conclusions
Revaccinations appear to be safe in patients with SARS-CoV-2 vaccine–induced SAT cases, while more evidence is needed regarding SARS-CoV-2 vaccine–induced GD.
Keywords: subacute thyroiditis, Graves’ disease, SARS-CoV-2, COVID-19, vaccine, revaccination
The pandemic of the new coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is still ongoing and has affected more than 250 million people, and 5 million were deceased by November 2021. A year after the pandemic began, Phase II/III trials of SARS-CoV-2 vaccinations had been published. Subsequently, COVID-19 immunization programs throughout the globe have started in early 2021, following the emergency use authorization of the vaccines. Inactivated whole virion SARS-CoV-2 vaccines (ie, CoronaVac developed by Sinovac Life Sciences) (1), vaccines using messenger RNA (mRNA) technology and lipid nanoparticle delivery systems (ie, BNT162b2 developed by Pfizer-BioNTech, mRNA-1273 developed by Moderna) (2, 3), and vaccines using nonreplicating recombinant adenovirus vector systems (i.e., ChAdOx1 nCoV-19 developed by Oxford-AstraZeneca) (4) are currently available and were all found effective and safe in clinical trials.
It has been hypothesized that SARS-CoV-2 might cause a wide range of autoimmune/autoinflammatory diseases via molecular mimicry, dysregulated immunity in response to COVID-19, or other as-yet-undiscovered pathways (5). Antibodies against the SARS-CoV-2 spike protein have been shown to cross-react with various human tissue proteins, including thyroid peroxidase (6). It has been postulated that vaccines against SARS-CoV-2 may also induce autoimmune reactions (5). mRNA and adenovirus-vectored vaccines encode (3, 4), and the inactivated SARS-CoV-2 vaccines contain (1) the SARS-CoV-2 spike protein, the primary target of neutralizing antibodies generated during natural infection. Therefore, cross-reactivity of the spike protein with thyroid antigens following vaccinations seems possible. Moreover, it has also been proposed that adjuvants found in vaccines might induce autoimmune/inflammatory syndrome in genetically susceptible individuals (7). Various autoimmune/autoinflammatory events linked to COVID-19 immunization have recently been documented in the literature, corroborating these findings (8, 9). Among all, cases of subacute thyroiditis (SAT) (10-26) have been most commonly encountered in daily practice, and Graves’ disease has also been reported (20, 25-34). However, active debate continues among physicians about managing such side effects, particularly the administration and timing of repeated or booster doses of the vaccines in such cases (35).
Therefore, we present the long-term clinical follow-up of SAT and Graves’ disease cases attributable to SARS-CoV-2 vaccinations and the effects of repeat SARS-CoV-2 vaccinations on disease outcomes.
Material and Methods
Patients and Study Design
Patients diagnosed with SAT or Graves’ disease between January and October 2021 who developed symptoms within 4 weeks of receiving the SARS-CoV-2 vaccinations were included in the study. Patients with <4 weeks of follow-up and positive for the polymerase chain reaction test for COVID-19 were excluded. Patients with a history of an upper respiratory infection in the previous 3 months were also precluded.
The American Thyroid Association guidelines of 2016 were followed to diagnose SAT and Graves’ disease (36). Patients were evaluated according to (1) clinical presentation and physical examination findings; (2) thyroid function tests including thyroid-stimulating hormone (TSH), free thyroxine (fT4), and free triiodothyronine (fT3), as well as serum thyroglobulin levels; (3) thyroid autoantibodies including antithyroglobulin antibodies (TgAb), antithyroid peroxidase antibodies (TPOAb) and thyroxine receptor antibodies (TRAb); (4) serum markers of inflammation; (5) ultrasonography findings; and (6) radioactive iodine uptake (RAIU) measurements when available.
Symptoms consistent with SAT such as neck pain, fever, and fatigue; elevated inflammatory markers such as erythrocyte sedimentation rate, C-reactive protein, or white blood cell count; typical thyroid ultrasonography/color flow Doppler ultrasonography findings showing the inhomogeneous hypoechoic area(s) with decreased blood flow; and low RAIU were used when diagnosing SAT.
Differential diagnosis of Graves’ disease was based on symptoms consistent with hyperthyroidism such as palpitations, sweating, fine tremor, and weight loss; thyroid function tests including fT3 showing overt or subclinical hyperthyroidism; positivity of thyroid autoantibodies; characteristic thyroid ultrasonography/color flow Doppler ultrasonography findings such as diffusely enlarged thyroid gland with increased vascularization; and increased or normal RAIU measurements.
Treatment modalities in SAT cases were determined according to the current guideline (36), the literature (37, 38), and local experience. Patients were followed without medication if they did not complain of significant neck discomfort. Patients with mild neck pain were initially treated with nonsteroid anti-inflammatory drugs (NSAIDs), but the treatment was changed to glucocorticoids if the pain did not disappear over several days. Glucocorticoid therapy was administered to patients with moderate to severe neck pain, usually at the dose of 16 mg methylprednisolone or equivalent. Then glucocorticoid treatment was tapered to a low enough dose to relieve the pain. All patients with thyrotoxic symptoms such as palpitations and distal tremors were treated with beta-adrenergic blockers.
Remission in SAT patients was defined as the absence of symptoms, discontinuation of medical treatment, normalization of both thyroid function tests and laboratory indicators of inflammation, and resolution of ultrasonography findings. Remission in Graves’ disease patients was defined as the absence of symptoms, normalization of thyroid function tests, and cessation of antithyroid medical treatment for at least 1 year (36).
Demographic features, history of thyroidal disease or surgery, and personal or family history of any autoimmune disorders were obtained. The type and dosing of SARS-CoV-2 vaccinations associated with SAT or Graves’ disease were documented. Period of time from vaccination to development of symptoms and from symptom onset to diagnosis, as well as the type and duration of medical treatment, were all recorded. Time since symptom onset to remission was noted in patients who were in remission when this manuscript was being written. It was documented whether revaccination induced an exacerbation or recurrence in SAT/Graves’ disease. In addition, the literature including cases with SARS-CoV-2 vaccine–induced SAT or Graves’ disease was thoroughly reviewed.
The quantitative determination of TSH, fT4, and fT3 were performed by chemiluminescent immunometric assays, employing the Unicell DxI-800 Access Immunoassay Systems, according to the manufacturer’s instructions (Beckman Coulter GmBH, Krefeld, Germany). The functional sensitivity of TSH measurement was 0.01 to 0.02 mIU/L. The analytical sensitivity of TSH measurement was 0.003 mIU/L. Thyroglobulin was measured using Siemens Immulite 2000/2000XPi assay (Siemens Healthcare Diagnostics Inc., USA). TgAb and TPOAb titers were measured using the Unicel Dxl-800 Access TPO Antibody and Thyroglobulin Antibody II assays, respectively (Beckman Coulter GmBH, Krefeld, Germany). TRAb titer was measured using Roche Elecsys Anti-TSHR assay (Roche catalog no. 04388780190; RRID: AB_2801453).
Results
The data of 19 patients from an area where 80% of the adult population received at least 2 doses of the SARS-CoV-2 vaccine were analyzed in this cohort. Fifteen of the patients were diagnosed with SARS-CoV-2 vaccine–induced SAT. The remaining 4 patients were diagnosed with Graves’ disease.
Patients With Subacute Thyroiditis
Clinical characteristics
Table 1 summarizes the clinical features of SARS-CoV-2 vaccine–induced SAT cases. The laboratory results and imaging findings of the patients were presented in Table 2. The median age of the patients was 46, with a range of 34 to 72. All subjects but 1 were female. Six patients had a history of thyroid disease: 4 patients had nodular goiter, whereas 3 (Cases 2, 4, and 5) had been diagnosed with SAT 45 days to 2 months before vaccinations that caused the SAT to exacerbate or recur. In 3 of the cases, there was a personal or family history of an autoimmune disorder unrelated to the thyroid. Case 14 developed Graves’ disease during follow-up.
Table 1.
Characteristics of patients with SARS-CoV-2 vaccine–induced subacute thyroiditis and Graves’ disease
| Age, years, and sex | Diagnosis | Type of vaccine | Time from vaccination to symptom onset | Therapy | Time from symptom onset to remission | History of thyroid disease | Personal/ family history of autoimmunity | |
|---|---|---|---|---|---|---|---|---|
| Case 1 | 42, F | SAT | BNT162b2 | 4 days after first dose | NSAIDs, 1 week | 14 weeks | Nodular thyroid disease | None |
| Case 2 | 48, F | SAT | CoronaVac | 1 day after second dose | Prednisolone 5 mg/day → 10 mg/day, tapered down and discontinued, 4 weeks | 5 weeks | Subacute thyroiditis diagnosed 1 month before vaccination | Personal history of systemic lupus erythematosus |
| Case 3 | 47, F | SAT | BNT162b2 | 10 days after first dose | Occasional paracetamol | 13 weeks | None | None |
| Case 4 | 72, F | SAT | CoronaVac (×2), BNT162b2 (×2) | 15 days after second BNT162b2 dose | None | 5 weeks | Subacute thyroiditis | None |
| Case 5 | 50, M | SAT | CoronaVac | 1 day after first dose | NSAIDs, 2 weeks | 6 weeks | MNG, prior subacute thyroiditis | None |
| Case 6 | 61, F | SAT | CoronaVac | 15 days after second dose | MPZ 16 mg/day, tapered down and discontinued, 4weeks | 20 weeks | MNG | None |
| Case 7 | 36, F | SAT | CoronaVac | 4 days after second dose | MPZ 16 mg/day, tapered down and discontinued, 12 weeks | Not in remission | None | None |
| Case 8 | 38, F | SAT | CoronaVac | 7 days after second dose | None | 11 weeks | None | None |
| Case 9 | 38, F | SAT | BNT162b2 | 10 days after first dose | NSAIDs, 2 weeks | 4 weeks | None | None |
| Case 10 | 38, F | SAT | CoronaVac | 13 days after first dose, symptoms aggravated after second dose | Occasional paracetamol, → NSAID, 1 week during relapse | 12 weeks | None | Family history of systemic lupus erythematosus and Sjögren’s syndrome in mother |
| Case 11 | 43, F | SAT | BNT162b2 | 7 days after second dose | MPZ 16 mg/day, 1 week→ MPZ 8 mg/day, 1 week→ NSAID, 4 weeks | 11 weeks | None | None |
| Case 12 | 60, F | SAT | CoronaVac (×2), BNT162b2 | 3 days after BNT162b2 | None | Not in remission | MNG, history of subtotal thyroidectomy | Personal history of undifferentiated connective tissue disease |
| Case 13 | 46, F | SAT | BNT162b2 | Symptoms start at first dose, aggravate 5 days after second dose | NSAIDs→ MPZ 16 mg/day, tapered down and discontinued, 4 weeks | 18 weeks | None | None |
| Case 14 | 34, F | SAT | CoronaVac | 4 days after first dose | MPZ 16 mg/day, tapered down and discontinued, 22 weeks→ methimazole 5 mg/day | Not in remission | None | None |
| Case 15 | 71, M | SAT | CoronaVac (×2), BNT162b2 | 10 days after BNT162b2 | Prednisolone 20 mg/day | Not in remission | None | None |
| Case 16 | 40, F | GD | CoronaVac (×2) BNT162b2 | 2 days after BNT162b2 | Methimazole 10 mg/day | Not in remission | None | None |
| Case 17 | 29, M | GD | BNT162b2 | 15 days after first dose | None | 10 weeks | None | None |
| Case 18 | 43, F | GD | CoronaVac (×2) BNT162b2 | 9 days after BNT162b2 | Methimazole 15 mg/day for 8 weeks → methimazole 10 mg/day | Not in remission | Nodular goiter | Personal history of ankylosing spondylitis |
| Case 19 | 43, F | GD | BNT162b2 | 14 days after first dose | LT4 was discontinued | Hypothyroidism resurfaced at 20th week | Autoimmune thyroiditis (hypothyroid, 25 mcg/day LT4 replacement) | Personal history of Hashimoto’s thyroiditis and diabetes insipidus |
Abbreviations: F, female; GD, Graves’ disease; M, male; NSAIDs, nonsteroid anti-inflammatory drugs; MNG, multinodular goiter; MPZ, methylprednisolone; LT4, levothyroxine; NA, not available; SAT, subacute thyroiditis; ×2, 2 doses.
Table 2.
Basal laboratory results and imaging findings of the patients
| Thyroid function tests | Thyroid autoantibodies | Inflammatory markers | Thyroid US/color Doppler US | Thyroid scintigraphy/RAIU | |
|---|---|---|---|---|---|
| Case 1 | TSH: <0.015 mIU/L fT4: 51.4 pmol/L fT3: 11.44 pmol/L |
TPOAb: 0.3 IU/mL TgAb: 2 IU/mL |
ESR: 74 mm/hour CRP: 4.44 mg/dL WBC: 6300/µL |
Patchy heterogenous hypoechoic areas in the right lobe | Partially suppressed thyroid gland |
| Case 2 | TSH: 0.031 mIU/L fT3: 10.35 pmol/L |
TPOAb: 0.5 IU/mL TgAb:183.6 IU/mL |
ESR: 48 mm/hour CRP: 5.8 mg/dL WBC: 8000/µL |
Patchy heterogenous hypoechoic areas | NA |
| Case 3 | TSH: 0.54 mIU/L fT4: 13.42 pmol/L fT3: 4.69 pmol/L Tg: 41.1 ng/mL |
TPOAb: 5.8 IU/mL TgAb: <0.9 IU/mL |
ESR: 55 mm/hour CRP: 4.85 mg/dL WBC: 10 800/µL |
Patchy heterogenous hypoechoic areas | NA |
| Case 4 | TSH: 2.44 mIU/L fT4: 11.81 pmol/L fT3: 4.66 pmol/L Tg: 9.14 ng/mL |
TPOAb:<0.25 IU/mL | ESR: 10 mm/hour CRP: 0.77 mg/dL WBC: 6400/µL |
Patchy heterogenous hypoechoic area in the right lobe | NA |
| Case 5 | TSH: 0.127 mIU/L fT4: 11.40 pmol/L fT3: 4.74 pmol/L Tg: 145 ng/mL |
TPOAb: 2.9IU/mL TgAb: 7.9 IU/mL TRAb: 0.9 IU/mL |
ESR: 41 mm/hour CRP: 1.02 mg/dL WBC: 5600/µL |
Ill-edged heterogenous hypoechoic area in the right lobe (50 × 25 mm) | NA |
| Case 6 | TSH: 4.44 mIU/L fT4: 10.99 pmol/L fT3: 4.71 pmol/L |
TPOAb: 1.2IU/mL TgAb: <0.9 IU/mL |
07/2021 ESR: 34 mm/hour CRP: 1.16 mg/dL WBC: 5500/µL |
Patchy heterogenous hypoechoic areas | NA |
| Case 7 | TSH: 0.47 mIU/L fT4: 19.11 pmol/L fT3: 6.15 pmol/L Tg: 36.3 ng/mL |
TPOAb: 1.2 IU/mL TgAb: 10.9 IU/mL TRAb: <1.5 IU/mL |
ESR: 53 mm/hour CRP: 10.5 mg/dL WBC: 9900/µL |
Patchy heterogenous hypoechoic areas, decreased vascularization | NA |
| Case 8 | TSH: 0.018 mIU/L fT4: 26.10 pmol/L fT3: 6.99 pmol/L |
TPOAb: 4.1 IU/mL TgAb: <0.9 IU/mL TRAb: <1.5 IU/mL |
ESR: 44 mm/hour CRP: 0.3 mg/dL WBC: 7800/µL |
Patchy heterogenous hypoechoic areas, decreased vascularization | NA |
| Case 9 | TSH: <0.01 mIU/L fT4: 51.48 pmol/L fT3: 6.99 pmol/L |
TPOAb: 6.11 IU/mL TgAb: <0.9 IU/mL TRAb: 0.45 IU/mL |
ESR: 55 mm/hour CRP: 13.63 mg/dL WBC: 11 200/µL |
Patchy heterogenous hypoechoic areas | NA |
| Case 10 | TSH: 0.032 mIU/L Ft4: 12.23 pmol/L Tg: 58.8 ng/mL |
TPOAb: 1.2 IU/mL TgAb: <0.9 IU/mL |
ESR: 42 mm/hour CRP: 1.9 mg/dL WBC: 5800/µL |
Patchy heterogenous hypoechoic areas | NA |
| Case 11 | TSH: 0.01 mIU/L fT4: 37.7 pmol/L fT3: 10.9 pmol/L |
TPOAb: 28 IU/mL (n < 34) TgAb: 26 IU/mL (N < 115) TRAb: 0.8 IU/mL |
CRP: 42.9 mg/dL WBC: 6950/µL |
Patchy heterogenous hypoechoic areas, decreased vascularization | 24-hour RAIU: 1% |
| Case 12 | TSH: 0.6 mIU/L fT4: 14.0 pmol/L fT3: 4.6 pmol/L |
NA | ESR: 33 mm/hour CRP: 5.2 mg/dL WBC: 7550/µL |
Patchy heterogenous hypoechoic area in the middle part of left lobe | NA |
| Case 13 | TSH: 0.43 mIU/L fT4: 14.08 pmol/L fT3: 5.59 pmol/L |
TPOAb: 0.7 IU/mL TgAb < 0.9 IU/mL |
ESR: 60 mm/hour CRP: 1.70 mg/dL WBC: 6400/µL |
Patchy heterogenous hypoechoic areas | NA |
| Case 14 | At SAT diagnosis: TSH: 0.03 mIU/L fT4: 31.65 pmol/L fT3: 10.54 pmol/L Tg: 196 ng/mL |
At SAT diagnosis: TPOAb: 1.2 IU/mL TgAb: <0.9 IU/mL TRAb: <1.5 IU/mL At GD diagnosis: TRAb: 3 IU/mL |
At SAT diagnosis: ESR: 18 mm/hour CRP: 0.6 mg/dL WBC: 10 000/µL |
At SAT diagnosis: Patchy heterogenous hypoechoic areas, decreased vascularization At GD diagnosis: Diffuse heterogenous thyroid gland |
At GD diagnosis: 24-hour RAIU 39% |
| Case 15 | TSH: 0.038 mIU/L fT4: 17.27 pmol/L fT3: 4.56 pmol/L |
TPOAb: 4.7 IU/mL TgAb: <0.9 IU/mL TRAb: 0.82 IU/mL |
ESR: 67 mm/hour CRP: 3.65 mg/dL WBC: 14 100/µL |
Patchy heterogenous hypoechoic areas, decreased vascularization | NA |
| Case 16 | TSH: <0.015 mIU/L fT4: 27.92 pmol/L fT3: 8.79 pmol/L |
TPOAb:195.7 IU/mL TgAb: 7.1 IU/mL TRAb: 10.3 IU/mL |
ESR: 11 mm/hour CRP: 0.84 mg/dL |
Diffuse hyperplasia, increased vascularization | Diffusely increased radiotracer uptake |
| Case 17 | TSH: <0.015 mIU/L fT4: 12.15 pmol/L fT3: 7.19 pmol/L |
TPOAb: 0.7 IU/mL TgAb: <0.9 IU/mL TRAb: 0.97 IU/mL |
ESR: 18 mm/hour CRP: 0.8 mg/dL |
Diffuse hyperplasia, increased vascularization | 24-hour RAIU: 27% |
| Case 18 | TSH: 0.015 mIU/L fT4: 33.1 pmol/L fT3: 11.4 pmol/L |
TPOAb: 0.8 IU/mL TgAb: 1.8 IU/mL TRAb: 0.25 IU/mL |
ESR: 17 mm/hour CRP: 0.6 mg/dL |
Diffuse hyperplasia, increased vascularization | 24-hour RAIU: 61% |
| Case 19 | TSH: 0.01 mIU/L fT4: 25.5 pmol/L fT3: 7.8 pmol/L |
TPOAb:196 IU/mL (N < 34) TgAb:167 IU/mL (N < 115) TRAb: 1.9 IU/mL |
ESR: 6 mm/hour CRP: 0.3 mg/dL |
Diffuse hyperplasia, increased vascularization | 24-hour RAIU: 23% |
TSH normal range, 0.38-5.33 mIU/L. fT4 normal range, 7.86-14.41 pmol/L. fT3 normal range, 3.8-6 pmol/L. Tg normal range, 1.15-50 ng/mL. ESR normal range, 0-25 mm/h. CRP normal range, 0-0.8 mg/dL. WBC normal range, 4100-11 200/µL. TPOAb normal range, 0-9 IU/mL (unless specified otherwise in the table). TgAb normal range, 0-4 IU/mL (unless specified otherwise in the table). TRAb normal range, <1.5 IU/mL. 24-hour RAIU normal range, 15-35%.
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; fT3, free triiodothyronine; fT4, free thyroxine; NA, not available; RAIU, radioactive iodine uptake; Tg, thyroglobulin; TgAb, antithyroglobulin antibody; TPOAb, antithyroid peroxidase antibody; TRAb, thyroid-stimulating hormone receptor antibody; TSH, thyroid-stimulating hormone; WBC US, ultrasound;, white blood cell.
Clinical presentation
The most frequent symptom was neck pain (93.3%), followed by palpitations (46.7%), myalgia (40%), fatigue (33.3%), and fever (33.3%). BNT162b2 was the vaccination most commonly related to SAT (n = 8, 53.3%). The median duration of time from vaccination to symptom onset was 7 days with a range of 1 to 15 days. The median diagnostic delay was 3 weeks, with a maximum of 13 weeks. Only 3 patients were diagnosed within 1 week of the onset of the symptoms (Table 1). The fact that these 3 patients were healthcare professionals may have facilitated the diagnosis.
Treatment and Outcomes
Seven patients (46.7%) were initially given glucocorticoids (n = 5, oral methylprednisolone; n = 2, oral prednisone), 4 (26.7%) were given NSAIDs alone (mostly indomethacin), and 4 (26.7%) were followed without medical treatment. Oral methylprednisolone and prednisone were initiated at maximal doses of 16 mg and 20 mg per day. All patients with symptomatic tachycardia were given propranolol at doses ranging from 40 to 80 mg/day. The median duration of medical treatment was 26.5 days (range 7-165 days); meanwhile, glucocorticoids were tapered almost weekly. At the time this paper was being written, 10 (66.7%) patients were in remission. The median duration of time from symptom onset to remission was 11.5 weeks, with a range of 4 to 20 weeks (Table 1).
Effect of repeat vaccinations on vaccine-induced SAT
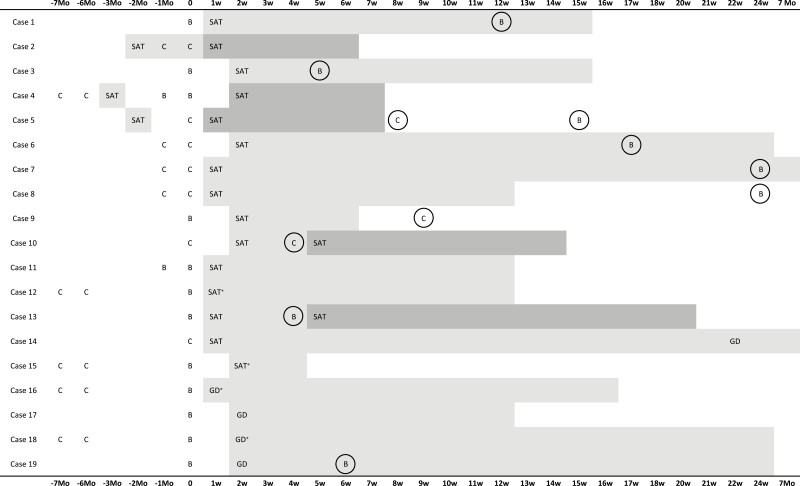
A total of 9 patients received repeat doses of SARS-CoV-2 vaccines following a diagnosis of SARS-CoV-2 vaccine–induced SAT. Figure 1 depicts the timing and types of repeat vaccinations. SAT did not recur in 7 cases (78%). However, in 2 patients (Cases 10 and 13), symptoms consistent with SAT that began after the first dose of vaccines (CoronaVac and BNT162b2, respectively) intensified after the second dose. The second doses of vaccines were administered while Case 10 was receiving occasional paracetamol and Case 13 required no medication for her neck discomfort. The second vaccination in both patients worsened the symptoms, necessitating medications (Table 1). Besides, in fear of a recurrence, 1 patient (Case 9) (Table 1, Fig. 1) received her second vaccination 1 month later than indicated as CoronaVac instead of BNT162b2, yet no recurrence was observed.
Figure 1.
SARS-CoV-2 vaccinations, as well as the onset and duration of disease in patients with SARS-CoV-2 vaccine–induced subacute thyroiditis (SAT) and Graves’ disease (GD), are illustrated in this timeline. SAT represents the onset of symptoms in patients with SAT, and GD represents the onset of symptoms in patients with GD. t0 illustrates the time of the administration of SARS-CoV-2 vaccination associated with thyroiditis. Mild gray indicates the time since remission or the follow-up period for patients who have not yet reached remission (*). Dark grey represents an exacerbation/recurrence in SAT. Repeat SARS-CoV-2 vaccinations that were received after SARS-CoV-2 vaccine–induced thyroiditis are circled. Abbreviations: B, BNT162b2; C, CoronaVac; Mo, month(s); w, week(s).
Results of patients with an episode of SAT prior to SARS-CoV-2 vaccinations
Before the SARS-CoV-2 vaccination, 3 patients (Cases 2, 4, and 5) had an episode of classical SAT, and COVID-19 vaccination resulted in a recurrence of SAT. Figure 1 presents the timings of previous SAT episodes as well as the vaccines that induced recurrences.
Case 2 was diagnosed with classical SAT 15 days before the first dose of CoronaVac and was being treated with prednisone 5 mg daily. The first dose of CoronaVac did not affect the course of SAT; however, 2 days after the second dose, patient’s symptoms deteriorated, necessitating an increase in prednisone dose to 10 mg per day.
Case 4, who received 2 doses of CoronaVac, was diagnosed with classical SAT 3 months after the second dose of the vaccine, which was not considered vaccine-induced SAT. SAT resolved completely after 1 month of glucocorticoid treatment. Two months following the resolution of classical SAT, the patient received the first booster dose of BNT162b2 and did not have any recurrent SAT symptoms. However, her symptoms relapsed after the second booster dose (Fig. 1). Thyroid function tests and laboratory markers of inflammation were normal, but thyroid ultrasound revealed a patchy hypoechoic heterogeneous area in the right lobe of the thyroid gland. She had only slight neck pain requiring no medication. Ultrasonography findings improved completely after 5 weeks.
Case 5 developed classical SAT 3 months before receiving the first dose of CoronaVac, which completely resolved within 2 months. Five days after the vaccination, he applied with fever and chills and was diagnosed with SAT relapse, which resolved after 2 weeks of NSAIDs. The patient postponed the second dose of the CoronaVac vaccine for 2 weeks because he was worried that his symptoms might return. However, he did not have any symptoms suggestive of SAT recurrence, neither after the second dose of CoronaVac nor a booster dose of BNT162b2 vaccines (Fig. 1). His thyroid function tests and ultrasonographic examination of the thyroid gland were normal 1 week following both vaccinations.
Graves’ disease following subacute thyroiditis
A 34-year-old female developed SAT 4 days after the first dose of CoronaVac. Her symptoms and ultrasonography examination of the thyroid gland were compatible with SAT, and TPOAb, TgAb, and TRAb tests were all negative. Her symptoms improved rapidly with oral methylprednisolone 16 mg/day but returned when the dose was reduced to 4 mg/day. Only after 5 months was the patient able to tolerate the withdrawal of glucocorticoid therapy. Her thyroid hormone levels, on the other hand, have remained elevated. The thyroid gland was found to be diffusely enlarged and vascularized on a repeat ultrasonographic scan. Her TRAb levels also became detectable (3 mIU/mL, normal range: <1.75), and an increased RAIU was found. There was no sign of thyroid ophthalmopathy. She was diagnosed with Graves’ disease, and methimazole 5 mg/day was initiated. The patient is still being followed.
Patients With Graves’ Disease
Clinical characteristics
The clinical characteristics of 4 patients (3 female, 1 male) diagnosed with Graves’ disease related to SARS-COV-2 vaccination are presented in Table 1. The laboratory results and imaging findings of the patients were presented in Table 2. The median age of the patients was 41.5, ranging from 29 to 43. Two patients had a history of thyroidal disease: 1 had nodular goiter, and 1 had primary hypothyroidism associated with Hashimoto’s disease. In addition, Case 18 had a history of ankylosing spondylitis, and Case 19 had idiopathic central diabetes insipidus diagnosed at the age of 26.
Clinical presentation
The median duration of time from vaccination to symptom onset was 11.5 days, with a range of 3 to 15 days. All patients had palpitations as a symptom, while sweating was evident in 2 of them. Graves’ disease developed after receiving BNT162b2 vaccinations in all cases—2 after the first dose and 2 after a booster dose following 2 doses of CoronaVac vaccinations (Fig. 1).
Treatment and outcomes
Cases 16 and 18 were administered methimazole, while the other 2 patients required no treatment (Table 1). Levothyroxine replacement (25 mcg/day) was suspended in Case 19 (Table 1), the patient with a history of Hashimoto’s, for 20 weeks while thyroid function tests were evaluated monthly. The patient had a slightly elevated TRAb level at the time of Graves’ disease diagnosis, and RAIU measurement was in the normal range (Table 2). Levothyroxine replacement was restarted at the 20th week of follow-up, when hypothyroidism resurfaced. Cases 17 and 19, who have not required antithyroid medication, were in remission when this paper was being written, while Cases 16 and 18 were still on methimazole (Fig. 1).
Effect of repeat vaccinations on vaccine-induced Graves’ disease
Case 19 received a repeated dose of BNT162b2 vaccine, and no relapse in symptoms or signs of Graves’ disease was observed (Table 1, Fig. 1).
Discussion
We evaluated the clinical characteristics, management, and follow-up of patients with SARS-COV-2 vaccine–induced SAT and Graves’ disease. We were able to determine the time to remission with longer follow-up and, at least in part, evaluate whether additional vaccine doses are safe in these patients.
There has been no evidence on the safety of SARS-CoV-2 revaccinations regarding thyroiditis relapse or exacerbation to date. Both patients and physicians may be hesitant to administer repeat doses (32, 33) as in our 2 patients. In our cohort, there was no exacerbation of SAT symptoms in two thirds of the cases who received a repeat dose of SARS-CoV-2 vaccination. In 2 of our cases of SARS-CoV-2 vaccine–induced SAT (Cases 10 and 13) (Table 1) occurring after the first vaccination; however, symptoms worsened following the second dose. Nonetheless, they were in remission shortly after SAT exacerbation (Fig. 1). There was only 1 case report describing a similar progression (25). In addition, SARS-CoV-2 vaccines induced exacerbation or recurrence of symptoms in 3 of our patients who had been diagnosed with classical SAT before vaccination (Cases 2, 4, and 5) (Fig. 1), which has never been documented in the literature before. All 3 cases were in remission shortly after SAT exacerbation/recurrence. SARS-CoV-2 vaccination/revaccinations appear safe in patients who develop SARS-CoV-2 vaccine–induced SAT or in patients with a history of classical SAT. The group with a history of classical SAT before SARS-CoV-2 vaccinations that relapsed after the vaccine and the group that developed SAT after the first dose of vaccine and deteriorated with revaccination had a maximum of 2 SAT episodes. Revaccinations have not induced another SAT episode in these patients. None of the patients has experienced severe side effects that could be associated with revaccinations. Therefore, physicians should closely follow their patients with a history of SAT and inform and reassure them about disease recurrences and revaccination.
BNT162b2 was the type of vaccination that precipitated thyroiditis in all patients with Graves’ disease and more than half of the patients with SAT. Cases 16 and 18 developed Graves’ disease after a BNT162b2 booster dose administered 6 months after 2 doses of CoronaVac (Table 1, Fig. 1). Given that prior CoronaVac vaccinations had not induced thyroiditis in either patient and that all reported cases of SARS-CoV-2 vaccine–induced Graves’ disease in the literature were associated with either mRNA or adenovirus-vectored type vaccines (Table 3), inactivated SARS-CoV-2 vaccines seem to be safe in terms of inducing Graves’ disease. Nonetheless, it is essential to determine the underlying reasons for the lack of documented cases of inactivated SARS-CoV-2 vaccine–induced Graves’ disease and the relative paucity of SARS-CoV-2 vaccine–induced SAT cases. One possible explanation might be that mRNA and adenovirus-vectored type vaccines have higher immunogenicity than inactivated SARS-CoV-2 vaccines (39).
Table 3.
Characteristics of patients with SARS-CoV-2 vaccine–induced thyroid disease reported in the literature
| Age, years, and sex | Type of vaccine | Time from vaccination to symptom onset | Diagnosis | Treatment | Time to remission | Relapse after revaccination | History of thyroid disease | Personal or family history of autoimmune diseases | |
|---|---|---|---|---|---|---|---|---|---|
| Bornemann et al (11) | 26, F | Adenovirus-vectored (1st) | 2 days | SAT | NSAIDs → Prednisone 50 mg/day | 6 months | NA | None | NA |
| 49, F | mRNA-1273 (1st) | 7 days | SAT | NSAIDs for 2 weeks → Prednisone 20 mg/day | NA | NA | None | NA | |
| Şahin Tekin et al (12) | 67, M | Inactivated whole virion (2nd) | 15 days | SAT | NSAIDs | 2 months | NA | MNG | NA |
| Oyibo(13) | 55, F | Adenovirus-vectored (1st) | 21 days | SAT | NSAIDs | 6 weeksa | NA | None | None |
| Schimmel, et al (14) | 57, F | BNT162b2 (2nd) | 1 day | SAT | NSAIDs → Prednisone | NA | NA | None | None |
| Soltanpoor and Norouzi (15) | 34, F | Inactivated whole virion (1st) | 5-7 days | SAT | Prednisone 15 mg/day, tapered and discontinued in 6 weeks | 7 weeks | NA | None | NA |
| Das et al (16) | 47, F | Adenovirus-vectored (1st) | 14 days | SAT | None | 8 weeks | NA | NA | NA |
| Saygili and Karakilic (17) | 38, F | Inactivated whole virion (2nd) | 14 days | SAT | NSAIDs | 1 montha | NA | None | None |
| Patel et al (18) | 48, M | NA (2nd) | 7 days | SAT | NSAID + prednisone | NA | NA | NA | NA |
| Sigstad et al (19) | 30, F | BNT162b2 (1st) | 6 days | SAT | Unilateral thyroidectomy with a preliminary diagnosis of PTC | NA | NA | NA | NA |
| Lee et al (20) | 39, F | Adenovirus-vectored (2nd) | 4 days | SAT | NA | NA | NA | NA | NA |
| 73, F | Adenovirus-vectored (1st) | 11 days | SAT | NA | NA | NA | NA | NA | |
| 39, M | Adenovirus-vectored | 14 days | GD and SAT | NA | NA | NA | NA | NA | |
| Chatzi et al (21) | 35, F | mRNA (1st) | 12 days | SAT | Prednisolone | NA | NA | NA | HT in mother |
| 32, F | mRNA (2nd) | 4 days | SAT | Prednisolone | NA | NA | NA | HT in mother | |
| Sioloset al (22) | 51, F | BNT162b2 (1st dose) | 4 days | SAT | MPZ 16 mg/day | 8 weeks | NA | None | None |
| 39, F | Adenovirus-vectored | NA | SAT | None | 8 weeks | NA | None | HT in mother | |
| Kyriacou et al (23) | 40, F | BNT162b2 (2nd) | 1 day | SAT | Prednisolone 40 mg/day | NA | NA | None | NA |
| Jeeyavudeen et al (24) | NA, F | BNT162b2 (2nd) | 14 days | SAT | NSAIDs | NA | NA | None | None |
| Ravenet al (25) | 35, F | BNT162b2 (1st) | 4 days | SAT | NA | NA | Worsens w/2nd dose | Subtotal thyroidectomy | NA |
| Pujol et al (26) | 38, F | mRNA-1273 (1st) | 8 days | SAT | Prednisone, NSAIDs | NA | NA | NA | NA |
| 32, M | BNT162b2 (1st) | 10 days | SAT | None | 8 weeksa | NA | NA | T1D | |
| Vera-Lastraet al (27) | 40 | BNT162b2 | 2 days | GD | Methimazole 10 mg/day | NA | NA | None | None |
| 28 | BNT162b2 | 3 days | GD | Methimazole 10 mg/day | NA | NA | None | None | |
| Lee et al (20) | 46, F | Adenovirus-vectored (1st) | 1 day | GD | NA | NA | NA | NA | NA |
| 73, F | Adenovirus-vectored (2nd) | 14 days | GD | NA | NA | NA | NA | NA | |
| 34, M | Adenovirus-vectored | 14 days | GD relapse | NA | NA | NA | NA | NA | |
| Zettinig and Krebs (28) | 71, F | BNT162b2 (2nd) | 15 days | GD relapse | Anti-thyroid medication | NA | NA | GD, cured since 2004 | NA |
| 46, M | BNT162b2 (1st) | 15 days | GD | Anti-thyroid medication | NA | NA | None | NA | |
| Sriphrapradang (29) | 30, F | Adenovirus-vectored (booster) | 4 days | GD worsening | Methimazole dose increased | NA | NA | GD since 2018 | GD |
| Patrizio et al (30) | 52, M | BNT162b2 (2nd) | 28 days | GD | Methimazole | NA | NA | NA | Vitiligo, T1D |
| Rubinstein (31) | 50, F | BNT162b2 (1st) | 3 days | GD relapse | Teprotumumab | NA | NA | GD, cured since 2010 | NA |
| di Filippo et al (32) | 32, M | Adenovirus-vectored (2nd) | 10 days | GD | Methimazole 15 mg/day → PTU 150 mg/day | NA | NA | None | None |
| 35, M | Adenovirus-vectored (1st) | 5 days | GD | Methimazole 15 mg/day | NA | Counseled not to receive the 2nd dose | None | None | |
| Yamamoto et al (33) | 64, F | BNT162b2 (1st) | 4 days | GD | Methimazole | NA | No relapse w/2nd dose | None | NA |
| Pierman et al (34) | 34, F | BNT162b2 (1st) | 10 days | GD relapse | Methimazole 20 mg/day | NA | Worsens w/2nd dose | GD, cured since 2014 | NA |
| Raven et al (25) | 35, F | Adenovirus-vectored (1st) | 5 days | GD | Carbimazole | NA | NA | NA | Hyperthyroidism |
| Pujol et al (26) | 38, F | BNT162b2 (1st) | 12 days | GD | Methimazole | NA | NA | NA | NA |
Abbreviations: F, Female. M, Male. SAT, Subacute thyroiditis; GD, Graves’ disease; NSAIDs, Non-steroid anti-inflammatory drugs; MPZ, Methylprednisolone; PTU, Propylthiouracil; MNG, Multinodular goiter; RAI, Radioactive iodine; PTC, Papillary thyroid carcinoma; HT, Hashimoto’s thyroiditis; T1D, Type 1 diabetes; NA, Not available; mRNA, messenger RNA;
aPatients became hypothyroid.
Two patients with SARS-CoV-2 vaccine–induced Graves’ disease, 1 in our cohort (Case 19) and 1 from the literature (34), received a repeat dose of mRNA vaccine. The latter case had worsening symptoms with revaccination whereas the patient in our cohort did not. Further data are needed to comment on repeat doses in patients with SARS-CoV-2 vaccine–induced Graves’ disease. All patients in our SARS-CoV-2 vaccine–induced Graves’ disease cohort were new-onset; however, there were 5 reported Graves’ recurrence/exacerbation cases in the literature, with 2 developing orbitopathy (Table 3) (20, 28, 29, 31, 34).
Most patients with SARS-CoV-2 vaccine–induced SAT and Graves’ disease that were described in the literature, including our series, were female, possibly reflecting women’s susceptibility to autoimmune/autoinflammatory disorders. The median age of previously published SARS-CoV-2 vaccine–induced SAT cases was younger than ours whereas that of Graves’ disease was similar. Symptoms initiated within 2 weeks following vaccination in most of the patients, regardless of the type of the vaccine. Clinical manifestations were also similar independent of vaccine type, except that no cases of Graves’ disease associated with inactive SARS-CoV-2 vaccines were reported. The median time from symptom onset to diagnosis was 3 weeks in our cohort, which was not reported previously. Clinicians should consider SARS-CoV-2 vaccine–induced thyroiditis earlier in patients developing symptoms compatible with SAT or Graves’ disease after vaccinations, especially if cardiac risk factors are present (33).
Unlike the clinical course of classical SAT, lower percentage of SARS-CoV-2 vaccine–induced SAT cases required a treatment (10). Data on the duration of medical therapy were generally unavailable in the literature, and remission was documented in 8 patients. In our cohort, the median duration of medical treatment was shorter in cases with SARS-CoV-2 vaccine–induced SAT compared to classical SAT (10). The recovery time was comparable to that of classical SAT cases, although it was longer than SAT caused by other vaccines such as influenza and hepatitis B vaccinations (10).
When writing the manuscript, no results were available for time to remission for the cases of SARS-CoV-2 vaccine–induced Graves’ disease who needed medical treatment. There are also no available data in the literature regarding recovery time in these cases. Notably, 2 patients in our Graves’ disease cohort required no antithyroid medication. Case 17 had a negative TRAb test, but an increased vascularization in the thyroid gland color Doppler ultrasonography and a RAIU measurement within the normal range were consistent with Graves’ disease (Table 2), and hyperthyroidism remitted spontaneously within a short time. Case 19, who had a history of hypothyroidism associated with Hashimoto’s thyroiditis, also had a mild SARS-CoV-2 vaccine–induced Graves’ disease course with a slightly increased TRAb level (Tables 1 and 2). On the other hand, only a slight elevation in fT3 level in Case 17, a relatively high fT4/fT3 ratio and a mildly positive TRAb level in Case-19, and increased vascularization on Doppler ultrasound in both cases cannot exclude the diagnosis of destructive thyroiditis. Therefore, as with postvaccination SAT, the clinical course of postvaccination Graves’ disease might be mild and short-term in some cases or transient destructive thyroiditis or a temporary worsening of existing autoimmune thyroiditis may have complicated patients who developed postvaccination thyrotoxicosis. Further data are required to support these observations.
After nearly 6 months of follow-up, 1 of our patients with SARS-CoV-2 vaccine–induced SAT developed Graves’ disease (Case 14), which had previously been described in the course of SAT (40). As the presence of thyroid autoantibodies was suggested to be a consequence of an immune response against thyroid antigens being released due to gland injury during SAT (41), the release of thyroid antigens during SARS-CoV-2 vaccine–induced SAT might have triggered the development of future Graves’ in our case. In the literature, 1 case with concurrent SAT and Graves’ disease associated with an adenovirus-vectored SARS-CoV-2 vaccine was reported (20), which is a very unusual occurrence (42).
In conclusion, all SARS-CoV-2 vaccines can cause SAT, either as a new-onset disease or a recurrence of a previously diagnosed classical SAT. However, only mRNA and adenovirus-vectored SARS-CoV-2 vaccine–induced new-onset or relapsed Graves’ disease cases have been reported to date. A longer follow-up period allowed us, at least in part, to determine the duration of medical treatment and recovery time and also to gain insight into the safety of repeated vaccinations regarding thyroiditis relapse. Medical treatment was needed in fewer patients with SARS-CoV-2 vaccine–induced SAT cases than classical SAT, whereas the time to recovery was similar. Revaccinations appear to be safe in patients with SARS-CoV-2 vaccine–induced SAT cases, while more evidence is needed regarding SARS-CoV-2 vaccine–induced Graves’ disease. Given the potentially catastrophic consequences of COVID-19, the development of these adverse events should not discourage physicians from offering vaccinations against SARS-CoV-2, including repeat doses. Instead, it is essential to consider thyroiditis and check thyroid function tests in patients who develop symptoms that might be compatible with SAT or Graves’ disease soon after vaccination.
Acknowledgments
We thank the patients, whose written consent was obtained and who kindly agreed to cooperate with this study.
Financial Support
The authors report no external funding sources.
Author Contributions
S.H.O. and U.Ü. performed the literature search and drafted the manuscript. All authors (S.H.O., S.N.Ş., B.G.İ, A.G., T.E., and U.Ü.) contributed to the coordination of the patients’ care and read and approved the final manuscript.
Disclosure Summary
The authors have no conflict of interest to declare.
Statement of Ethics
Written consent was obtained from all patients included in this study.
Data Availability
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Jara A, Undurraga EA, Gonzalez C, et al. . Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):875-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polack FP, Thomas SJ, Kitchin N, et al. ; for the for the C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baden LR, Sahly HM E, Essink B, et al. . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Voysey M, Clemens SAC, Madhi SA, et al. . Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ehrenfeld M, Tincani A, Andreoli L, et al. . Covid-19 and autoimmunity. Autoimmun Rev. 2020;19(8):102597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shoenfeld Y, Agmon-Levin N. “ASIA” - autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011;36(1):4-8. [DOI] [PubMed] [Google Scholar]
- 8. Dotan A, Shoenfeld Y. Perspectives on vaccine induced thrombotic thrombocytopenia. J Autoimmun. 2021;121:102663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vuille-Lessard E, Montani M, Bosch J, Semmo N. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. J Autoimmun. 2021;123:102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iremli BG, Sendur SN, Unluturk U. Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: postvaccination ASIA syndrome. J Clin Endocrinol Metab. 2021;106(9):2600-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bornemann C, Woyk K, Bouter C. Case report: two cases of subacute thyroiditis following SARS-CoV-2 vaccination. Front Med (Lausanne). 2021;8:737142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Şahin Tekin M, Şaylisoy S, Yorulmaz G. Subacute thyroiditis following COVID-19 vaccination in a 67-year-old male patient: a case report. Hum Vaccin Immunother. 2021;17(11):4090-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oyibo SO. Subacute thyroiditis after receiving the adenovirus-vectored vaccine for coronavirus disease (COVID-19). Cureus. 2021;13(6):e16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schimmel J, Alba EL, Chen A, Russell M, Srinath R. Letter to the editor: thyroiditis and thyrotoxicosis after the SARS-CoV-2 mRNA vaccine. Thyroid. 2021;31(9):1440. [DOI] [PubMed] [Google Scholar]
- 15. Soltanpoor P, Norouzi G. Subacute thyroiditis following COVID-19 vaccination. Clin Case Rep. 2021;9(10):e04812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Das L, Bhadada SK, Sood A. Post-COVID-vaccine autoimmune/inflammatory syndrome in response to adjuvants (ASIA syndrome) manifesting as subacute thyroiditis. J Endocrinol Invest. 2022;45(2):465-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saygili ES, Karakilic E. Subacute thyroiditis after inactive SARS-CoV-2 vaccine. BMJ Case Rep. 2021;14(10):e244711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patel KR, Cunnane ME, Deschler DG. SARS-CoV-2 vaccine-induced subacute thyroiditis. Am J Otolaryngol. 2021;43(1):103211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sigstad E, Groholt KK, Westerheim O. Subacute thyroiditis after vaccination against SARS-CoV-2. Tidsskr Nor Laegeforen. 2021;141(2021-14). Doi: 10.4045/tidsskr.21.0554. [DOI] [PubMed] [Google Scholar]
- 20. Lee KA, Kim YJ, Jin HY. Thyrotoxicosis after COVID-19 vaccination: seven case reports and a literature review. Endocrine. 2021;74(3):470-472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chatzi S, Karampela A, Spiliopoulou C, Boutzios G. Subacute thyroiditis after SARS-CoV-2 vaccination: a report of two sisters and summary of the literature. Hormones (Athens). Published online October 22, 2021. Doi: 10.1007/s42000-021-00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siolos A, Gartzonika K, Tigas S. Thyroiditis following vaccination against COVID-19: report of two cases and review of the literature. Metabol Open. 2021;12:100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kyriacou A, Ioakim S, Syed AA. COVID-19 vaccination and a severe pain in the neck. Eur J Intern Med. 2021;94:95-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jeeyavudeen MS, Patrick AW, Gibb FW, Dover AR. COVID-19 vaccine-associated subacute thyroiditis: an unusual suspect for de Quervain’s thyroiditis. BMJ Case Rep. 2021;14(11):e246425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raven LM, McCormack AI, Greenfield JR. Letter to the editor from Raven: three cases of subacute thyroiditis following SARS-CoV-2 vaccine. J Clin Endocrinol Metab. Published online November 9, 2021. Doi: 10.1210/clinem/dgab822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pujol A, Gomez LA, Gallegos C, et al. . Thyroid as a target of adjuvant autoimmunity/inflammatory syndrome due to mRNA-based SARS-CoV2 vaccination: from Graves’ disease to silent thyroiditis. J Endocrinol Invest. Published online November 18, 2021. Doi: 10.1007/s40618-021-01707-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vera-Lastra O, Ordinola Navarro A, Cruz Domiguez MP, Medina G, Sanchez Valadez TI, Jara LJ. Two cases of Graves’ disease following SARS-CoV-2 vaccination: an autoimmune/inflammatory syndrome induced by adjuvants. Thyroid. 2021;31(9):1436-1439. [DOI] [PubMed] [Google Scholar]
- 28. Zettinig G, Krebs M. Two further cases of Graves’ disease following SARS-Cov-2 vaccination. J Endocrinol Invest. 2022;45(1):227-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sriphrapradang C. Aggravation of hyperthyroidism after heterologous prime-boost immunization with inactivated and adenovirus-vectored SARS-CoV-2 vaccine in a patient with Graves’ disease. Endocrine. 2021;74(2):226-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patrizio A, Ferrari SM, Antonelli A, Fallahi P. A case of Graves’ disease and type 1 diabetes mellitus following SARS-CoV-2 vaccination. J Autoimmun. 2021;125:102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rubinstein TJ. Thyroid eye disease following COVID-19 vaccine in a patient with a history Graves’ disease: a case report. Ophthalmic Plast Reconstr Surg. 2021;37(6):e221-e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. di Filippo L, Castellino L, Giustina A. Occurrence and response to treatment of Graves’ disease after COVID vaccination in two male patients. Endocrine. 2022;75(1):19-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamamoto K, Mashiba T, Takano K, et al. . A case of exacerbation of subclinical hyperthyroidism after first administration of BNT162b2 mRNA COVID-19 vaccine. Vaccines (Basel). 2021;9(10):1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pierman G, Delgrange E, Jonas C. Recurrence of Graves’ disease (a Th1-type cytokine disease) following SARS-CoV-2 mRNA vaccine administration: a simple coincidence? Eur J Case Rep Intern Med 2021;8(9):002807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iremli BG, Sendur SN, Unluturk U. Response letter to the editor from Raven: three cases of subacute thyroiditis following SARS-CoV-2 vaccine. J Clin Endocrinol Metab. Published online November 9, 2021. Doi: 10.1210/clinem/dgab823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ross DS, Burch HB, Cooper DS, et al. . 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343-1421. [DOI] [PubMed] [Google Scholar]
- 37. Kubota S, Nishihara E, Kudo T, Ito M, Amino N, Miyauchi A. Initial treatment with 15 mg of prednisolone daily is sufficient for most patients with subacute thyroiditis in Japan. Thyroid. 2013;23(3):269-272. [DOI] [PubMed] [Google Scholar]
- 38. Hepsen S, Akhanli P, Sencar ME, et al. . The evaluation of low- and high-dose steroid treatments in subacute thyroiditis: a retrospective observational study. Endocr Pract. 2021;27(6):594-600. [DOI] [PubMed] [Google Scholar]
- 39. Mukhopadhyay L, Yadav PD, Gupta N, et al. . Comparison of the immunogenicity & protective efficacy of various SARS-CoV-2 vaccine candidates in non-human primates. Indian J Med Res. 2021;153(1 & 2):93-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iitaka M, Momotani N, Hisaoka T, et al. . TSH receptor antibody-associated thyroid dysfunction following subacute thyroiditis. Clin Endocrinol (Oxf). 1998;48(4):445-453. [DOI] [PubMed] [Google Scholar]
- 41. Stasiak M, Lewinski A. New aspects in the pathogenesis and management of subacute thyroiditis. Rev Endocr Metab Disord. 2021;22(4):1027-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fang F, Yan S, Zhao L, Jin Y, Wang Y. Concurrent onset of subacute thyroiditis and Graves disease. Am J Med Sci. 2016;352(2):224-226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.