Abstract
Background
Non-pharmaceutical interventions (NPIs) are recommended for COVID-19 prevention. However, the effectiveness of NPIs in preventing SARS-CoV-2 transmission remains poorly quantified.
Methods
We conducted a test-negative design case-control study enrolling cases (testing positive for SARS-CoV-2) and controls (testing negative) with molecular SARS-CoV-2 diagnostic test results reported to California Department of Public Health between 24 February–12 November, 2021. We used conditional logistic regression to estimate adjusted odds ratios (aORs) of case status among participants who reported contact with an individual known or suspected to have been infected with SARS-CoV-2 (“high-risk exposure”) ≤14 days before testing.
Results
751 of 1448 cases (52%) and 255 of 1443 controls (18%) reported high-risk exposures ≤14 days before testing. Adjusted odds of case status were 3.02-fold (95% confidence interval: 1.75–5.22) higher when high-risk exposures occurred with household members (vs. other contacts), 2.10-fold (1.05–4.21) higher when exposures occurred indoors (vs. outdoors only), and 2.15-fold (1.27–3.67) higher when exposures lasted ≥3 hours (vs. shorter durations) among unvaccinated and partially-vaccinated individuals; excess risk associated with such exposures was mitigated among fully-vaccinated individuals. Cases were less likely than controls to report mask usage during high-risk exposures (aOR = 0.50 [0.29–0.85]). The adjusted odds of case status was lower for fully-vaccinated (aOR = 0.25 [0.15–0.43]) participants compared to unvaccinated participants. Benefits of mask usage were greatest among unvaccinated and partially-vaccinated participants, and in interactions involving non-household contacts or interactions occurring without physical contact.
Conclusions
NPIs reduced the likelihood of SARS-CoV-2 infection following high-risk exposure. Vaccine effectiveness was substantial for partially and fully vaccinated persons.
Keywords: SARS-CoV-2, non-pharmaceutical interventions, face masks, vaccination
SARS CoV-2 infection risk was greatest for unvaccinated participants when known or suspected exposures to cases occurred indoors or lasted ≥3 hours. Face mask usage when participants were exposed to a case reduced odds of infection by 50%.
Strategies aimed at preventing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission during contact between infectious and susceptible individuals have been critical to the mitigation of the coronavirus disease 2019 (COVID-19) pandemic. While vaccines effectively reduce individual risk of infection and severe disease [1–3], nonpharmaceutical interventions (NPIs) continue to be recommended in various circumstances. These include within populations ineligible for vaccination, in settings where vaccines remain inaccessible or underutilized, and in response to emergence of SARS-CoV-2 variants with increased transmissibility. Efforts to prevent transmission include social distancing and avoiding direct physical contact with nonhousehold members [4]; interacting with nonhousehold members outdoors [5]; and use of face coverings to filter virus-containing droplets and aerosols [6, 7].
However, evidence demonstrating the effectiveness of various NPIs in mitigating transmission risk remains limited [8, 9]. Understanding of exposures mediating SARS-CoV-2 transmission stems largely from anecdotal reports with unknown generalizability [10]. Additionally, many assessments of the effectiveness of NPIs have been ecological studies comparing COVID-19 incidence before and after implementation of multiple interventions [11–13], making it difficult to distinguish independent effects of each strategy [14]. While numerous studies demonstrate that face masks limit the quantity of virus shed into the environment by infectious individuals [15, 16], few have assessed real-world effectiveness of face masks in preventing SARS-CoV-2 infection [6]. Improved understanding of aspects of social contact that exacerbate or reduce risk of SARS-CoV-2 transmission are needed to guide intervention prioritization [17, 18].
To mitigate transmission of SARS-CoV-2, California mandated social distancing and wearing of facial coverings in spring 2020 and implemented a tiered system for closure and reopening of public places based on community-level measures of SARS-CoV-2 transmission and hospital utilization [19]. Statewide social distancing and mask mandates among vaccinated people in most public places and the tiered system were relaxed on 15 June 2021, when roughly 57% of eligible Californians were considered fully vaccinated [19, 20]. However, amid rising incidence of COVID-19 and increases in hospitalizations following emergence of the Delta (B.1.617.2) variant [21, 22], measures encouraging or requiring face masks in certain indoor settings regardless of vaccination status were reinstated on 17 July 2021 [23]. We initiated a retrospective, test-negative design case-control study to understand risk factors for SARS-CoV-2 infection in California and inform public health strategies [1]. Here, we address predictors of SARS-CoV-2 infection among participants who reported high-risk exposures, defined as social contact with an individual known or suspected to have been infected with SARS-CoV-2, within 2 weeks preceding participants’ SARS-CoV-2 tests.
METHODS
Design
California residents with confirmatory, molecular SARS-CoV-2 diagnostic test results reported to the California Department of Public Health between 24 February 2021 and 12 November 2021 with a recorded phone number were eligible for inclusion. Each day, trained interviewers called potential participants selected at random from all individuals with test results reported in the preceding 48 hours. Cases were persons with a positive molecular SARS-CoV-2 test result, and controls were persons with a negative result. We enrolled cases equally across 9 regions of the state (Supplementary Table 1). For each enrolled case, interviewers attempted to enroll 1 control matched to the case by age group, sex, region, and week of SARS-CoV-2 test from a list of ≥30 randomly selected controls who met these criteria. Individuals were eligible to enroll if they provided informed consent in English or Spanish and had not received a previous diagnosis of COVID-19 or positive test result for SARS-CoV-2 infection (molecular, antigen, or serological test). Additional sampling and enrollment details have been described elsewhere [1].
The State of California Health and Human Services Agency Committee for the Protection of Human Subjects approved the study protocol as public health surveillance.
Exposures
Trained interviewers administered a standardized phone-based questionnaire to assess exposures (Supplementary Text 1, Survey). This analysis included participants who reported they were potentially exposed to SARS-CoV-2 <14 days prior to their test through social contact with an individual known or suspected by the participant to have been infected with SARS-CoV-2 at the time of their interaction (“high-risk exposure”). Participants were asked to specify if they were aware that 1 or more of these individuals had been a confirmed case, based on receipt of a positive diagnostic test result for SARS-CoV-2 infection.
Among participants who reported exposure to a confirmed or suspected COVID-19 case during the 14 days prior to their test, interviewers systematically collected characteristics of the exposure including setting (any indoor exposure vs outdoor exposure only), duration (whether contact lasted ≥3 hours), whether the participant and the contact had any physical contact, whether the contact was a member of the participant’s household, and use of face coverings by the participant and the contact during the interaction(s).
Additionally, all study participants were asked to indicate their reasons for seeking SARS-CoV-2 testing, including any symptoms experienced in the 14 days preceding their test. Interviews also recorded participants’ self-reported history of visiting other locations, including restaurants, bars, coffee shops, retail shops, public gyms, salons, movie theaters, or worship services; participating in social gatherings; and using ride share services, public transportation, or air travel. Interviewers recorded the COVID-19 vaccination status of participants, including the manufacturer and dates of all doses received, and asked participants to describe their level of concern about the COVID-19 pandemic in the 14 days prior to seeking SARS-CoV-2 testing.
Statistical Analyses
To convey descriptive features of the enrolled sample, we summarized demographic attributes and exposure characteristics among participants enrolled in the study using proportions. Our primary inferential objective was to identify characteristics of high-risk exposure events associated with SARS-CoV-2 infection. We fit conditional logistic regression models to estimate adjusted odds ratios (aORs) and accompanying 95% confidence intervals (CIs) of various exposure attributes, comparing cases with controls. These included exposure setting (any indoor exposure vs outdoor-only exposure), exposure duration (any exposure ≥3 hours vs <3 hours), whether the exposure involved a potentially infectious household member(s) (vs nonhousehold contact(s) only), the nature of exposure (any physical contact vs no physical contact), and mask usage by the participant or their contact during the entire interaction (vs mask usage by neither party). Models included interaction terms between each contact attribute and the vaccination history of the participant at the time of their test to assess effect modification. We considered participants tested >14 days after receipt of 2 doses of BNT162b2 (Pfizer/BioNTech) or mRNA-1273 (National Institutes of Health/Moderna) or 1 dose of JNJ-78436735 (Janssen Pharmaceutical Companies) to be fully vaccinated. Others reporting receipt of any COVID-19 vaccine doses before their test date were considered partially vaccinated.
To correct for differences in infection prevalence over time and across regions, independent of the specific exposures being analyzed, regression strata (ie, matching sets) were defined by the reopening tier of participants’ county of residence at the time of testing or, for the period after 15 June 2021 (when the tiered reopening system was retired), by participants’ month of SARS-CoV-2 testing. We further controlled for potential confounders including demographic variables (age, sex, and region), participants’ self-reported level of anxiety about the COVID-19 pandemic prior to seeking SARS-CoV-2 testing, and participants’ self-reported attendance at community settings (as listed above) that may have been associated with risk of SARS-CoV-2 exposure. As a sensitivity analysis, we repeated these primary analyses within only the subset of participants who reported contact with a confirmed case. To further verify that findings did not owe to confounding between risk-mitigating behaviors and test-seeking, we repeated the analyses within the subset of participants who cited contact with a COVID-19 case as a primary motivation for their decision to receive a test.
To identify determinants of the effectiveness or impact of mask usage in mitigating transmission, we also undertook secondary analyses estimating the aOR of mask usage among cases vs controls within subsets of participants who reported distinct types of high-risk exposures [24]. Consistent with our primary analyses, these included indoor and outdoor exposures, exposures lasting ≥3 hours and <3 hours, exposures to potentially infected individuals who were and were not members of participants’ households, and exposures with and without physical contact. We further estimated the aOR of mask usage separately among fully vaccinated and partially vaccinated or unvaccinated participants. Conditional logistic regression models for these analyses followed the framework described above and included interaction terms between each exposure characteristic and mask usage by participants or their contacts.
Last, we aimed to test the hypothesis that attributes of high-risk exposure including face mask usage predicted the severity of illness among SARS-CoV-2–infected individuals [25–27]. Here, we restricted our analytic sample to cases testing positive for SARS-CoV-2. As a measure of severity, we considered whether participants reported any type of consultation with a healthcare provider (eg, virtual or outpatient appointment, emergency room attendance, or hospitalization) in conjunction with testing. We estimated aORs of each exposure characteristic, comparing cases who received clinical care to those who did not, using conditional logistic regression models following the same framework described above. We conducted analyses in R software (version 3.6.1).
RESULTS
Descriptive Features of the Enrolled Sample
Between 24 February 2021 and 12 November 2021, we enrolled 2891 participants, including 1448 cases and 1443 controls. In total, 1006 participants, including 751 cases (52% of 1448) and 255 controls (18% of 1443), reported high-risk exposure within 14 days before testing, including 833 (83% of 1006) with confirmed and 173 (17% of 1006) with suspected exposure (Table 1, Supplementary Tables 2 and 3). Most participants reported that their high-risk exposure occurred within their household (55% of 847) or workplace (14% of 847) (Supplementary Table 4). A majority of these participants (788 of 1006; 78%) listed high-risk exposure as a motivation for testing. In total, 600 (60% of 1006) indicated that they experienced symptoms, and 319 (32% of 1006) cited symptoms as a primary motivation for testing (Supplementary Table 5).
Table 1.
Descriptive Attributes of Participants Reporting High-Risk Exposures
| Participant Attribute | All Participants | Casesa | Controlsb | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| N = 1006 | N = 751 | N = 255 | ||
| Age, years | ||||
| 0–6 | 37 (3.7) | 35 (4.7) | 2 (0.8) | |
| 7–12 | 61 (6.1) | 44 (5.9) | 17 (6.7) | |
| 13–17 | 67 (6.7) | 50 (6.7) | 17 (6.7) | |
| 18–29 | 299 (29.7) | 223 (29.7) | 76 (29.8) | |
| 30–49 | 352 (35.0) | 245 (32.6) | 107 (42.0) | |
| 50–64 | 136 (13.5) | 110 (14.6) | 26 (10.2) | |
| ≥65 | 54 (5.4) | 44 (5.9) | 10 (3.9) | |
| Sex | ||||
| Male | 460 (45.7) | 343 (45.7) | 117 (45.9) | |
| Female | 546 (54.3) | 408 (54.3) | 138 (54.1) | |
| Household income | ||||
| Less than $50 000 | 245 (24.4) | 187 (24.9) | 58 (22.7) | |
| $50 000–$100 000 | 232 (23.1) | 185 (24.6) | 47 (18.4) | |
| $100 000–$150 000 | 127 (12.6) | 79 (10.5) | 48 (18.8) | |
| More than $150 000 | 128 (12.7) | 88 (11.7) | 40 (15.7) | |
| Refuse | 170 (16.9) | 131 (17.4) | 39 (15.3) | |
| Not sure | 104 (10.3) | 81 (10.8) | 23 (9.0) | |
| Race/ethnicity | ||||
| Non-Hispanic White | 441 (45.7) | 331 (45.9) | 110 (44.9) | |
| Non-Hispanic Black | 45 (4.7) | 37 (5.1) | 8 (3.3) | |
| Hispanic (any race) | 253 (26.2) | 192 (26.6) | 61 (24.9) | |
| Asian | 83 (8.6) | 62 (8.6) | 21 (8.6) | |
| Native American | 17 (1.8) | 15 (2.1) | 2 (0.8) | |
| Native Hawaiian | 5 (0.5) | 5 (0.7) | 0 (0.0) | |
| More than 1 race | 122 (12.6) | 79 (11.0) | 43 (17.6) | |
| Refuse | 40 (4.0) | 30 (4.0) | 10 (3.9) | |
| Region of residencec | ||||
| Predominantly urban regions | ||||
| San Francisco Bay area | 108 (10.7) | 87 (11.6) | 21 (8.2) | |
| Greater Los Angeles area | 98 (9.7) | 76 (10.1) | 22 (8.6) | |
| Greater Sacramento area | 128 (12.7) | 99 (13.2) | 29 (11.4) | |
| San Diego and southern border | 96 (9.5) | 75 (10.0) | 21 (8.2) | |
| Predominantly rural regions | ||||
| Central Coast | 127 (12.6) | 89 (11.9) | 38 (14.9) | |
| Northern Sacramento Valley | 106 (10.5) | 80 (10.7) | 26 (10.2) | |
| San Joaquin Valley | 111 (11.0) | 78 (10.4) | 33 (12.9) | |
| Northwestern California | 116 (11.5) | 79 (10.5) | 37 (14.5) | |
| Sierras region | 116 (11.5) | 88 (11.7) | 28 (11.0) | |
| Vaccination statusd | ||||
| Unvaccinated | 649 (68.4) | 546 (75.3) | 103 (46.0) | |
| Partially vaccinated | 83 (8.7) | 56 (7.7) | 27 (12.1) | |
| Fully vaccinated | 217 (22.9) | 123 (17.0) | 94 (42.0) | |
| County reopening tiere | ||||
| Purple (most restrictive) | 201 (20.0) | 160 (21.3) | 41 (16.1) | |
| Red | 206 (20.5) | 166 (22.1) | 40 (15.7) | |
| Orange | 200 (19.9) | 165 (22.0) | 35 (13.7) | |
| Yellow (least restrictive) | 23 (2.3) | 17 (2.3) | 6 (2.4) | |
| After 15 June | 376 (37.4) | 243 (32.4) | 133 (52.2) | |
| Symptoms experienced | ||||
| No symptoms | 406 (40.4) | 206 (27.4) | 200 (78.4) | |
| At least 1 symptom | 600 (59.6) | 545 (72.6) | 55 (21.6) | |
| Level of anxiety about coronavirus disease 2019 | ||||
| Very anxious | 157 (15.6) | 108 (14.4) | 49 (19.2) | |
| Not very anxious | 849 (84.4) | 643 (85.6) | 206 (80.8) | |
Recent high-risk exposure is defined as reported contact with an individual known or suspected to have been infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at any time within the 14 days before participants were tested. Covariates with significant associations at a 2-sided P < .05 type 1 error rate include age (P = .01), income (P = .004), county reopening tier (P < .001), vaccination status (P < .001), and symptoms (P < .001), based on the χ2 test. Percentages presented in the table should be interpreted to represent the proportion of cases or controls with each characteristic and not their prevalence within the general population. Abbreviation: ref, reference.
Cases reporting high-risk exposure represent 51% of 1474 cases who enrolled in and successfully completed the study.
Controls reporting high-risk exposure represent 18% of 1447 controls who enrolled in and successfully completed the study.
We list counties grouped into each region in Supplementary Table 1.
We defined participants as fully vaccinated at the time of their test if >14 days had passed following receipt of a second dose of Pfizer/BioNTech BNT-162b2 or Moderna mRNA-1273 (108 cases, 686 controls) or a single dose of Jansen Pharmaceutical Companies JNJ-78436735 (15 cases, 8 controls). Participants who had received at least 1 dose of any coronavirus disease 2019 (COVID-19) vaccine but did not meet these criteria for fully vaccinated status were considered partially vaccinated (52 cases and 24 controls who received BNT162b2 or mRNA-1273; 4 cases and 3 controls who received JNJ-78436735). Participants who had not received any COVID-19 vaccine doses were considered unvaccinated.
The State of California implemented a tiered system of reopening to reduce risk of SARS-CoV-2 transmission in community settings. On 15 June 2021, California discontinued the tiered system, relaxed facial masking requirements in certain indoor settings, and allowed businesses to reopen without physical distancing restrictions. Numbers of participants enrolled by month (including after 15 June) are presented in Supplementary Table 3.
Among 1006 participants reporting high-risk exposure, 880 (87%) reported contact occurring indoors, 728 (72%) reported contact lasting ≥3 consecutive hours, 594 (59%) reported physical contact with the individual known or suspected to have been infected, and 559 (56%) indicated their contact was a household member. Participants who reported interactions occurring indoors, lasting ≥3 hours, or involving physical contact were generally more likely to have been enrolled after 15 June or to have resided in counties within less-restrictive reopening tiers at the time of their test than those who reported outdoor, shorter, or nonphysical contact (Table 2).
Table 2.
Attributes of Participants Reporting High-Risk Exposure With Differing Characteristics of Contact
| Participant Attribute | Relationship to Contact | Exposure Setting | Duration of Exposure | Nature of Contact | |||||
|---|---|---|---|---|---|---|---|---|---|
| Nonhousehold Member | Household Member | Outdoor Exposure Only | Any Indoor Exposure | <3 Hours | >3 Hours | Nonphysical Contact Only | Any Physical Contact | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| N = 559 | N = 444 | N = 91 | N = 880 | N = 252 | N = 728 | N = 363 | N = 594 | ||
| Age, years | |||||||||
| 0–6 | 12 (2.1) | 25 (5.6) | 1 (1.1) | 35 (4.0) | 3 (1.2) | 34 (4.7) | 2 (0.6) | 31 (5.2) | |
| 7–12 | 31 (5.5) | 30 (6.8) | 3 (3.3) | 53 (6.0) | 6 (2.4) | 53 (7.3) | 20 (5.5) | 36 (6.1) | |
| 13–17 | 31 (5.5) | 36 (8.1) | 8 (8.8) | 54 (6.1) | 6 (2.4) | 55 (7.6) | 18 (5.0) | 40 (6.7) | |
| 18–29 | 183 (32.7) | 115 (25.9) | 29 (31.9) | 258 (29.3) | 81 (32.1) | 208 (28.6) | 121 (33.3) | 163 (27.4) | |
| 30–49 | 195 (34.9) | 156 (35.1) | 33 (36.3) | 310 (35.2) | 95 (37.7) | 252 (34.6) | 131 (36.1) | 209 (35.2) | |
| 50–64 | 78 (14.0) | 57 (12.8) | 10 (11.0) | 123 (14.0) | 42 (16.7) | 91 (12.5) | 49 (13.5) | 83 (14.0) | |
| ≥65 | 29 (5.2) | 25 (5.6) | 7 (7.7) | 47 (5.3) | 19 (7.5) | 35 (4.8) | 22 (6.1) | 32 (5.4) | |
| Sex | |||||||||
| Male | 256 (45.8) | 204 (45.9) | 46 (50.5) | 395 (44.9) | 117 (46.4) | 334 (45.9) | 177 (48.8) | 259 (43.6) | |
| Female | 303 (54.2) | 240 (54.1) | 45 (49.5) | 485 (55.1) | 135 (53.6) | 394 (54.1) | 186 (51.2) | 335 (56.4) | |
| Household income | |||||||||
| Less than $50 000 | 146 (26.1) | 98 (22.1) | 21 (23.1) | 219 (24.9) | 62 (24.6) | 178 (24.5) | 93 (25.6) | 147 (24.7) | |
| $50 000–$100 000 | 121 (21.6) | 111 (25.0) | 17 (18.7) | 205 (23.3) | 54 (21.4) | 170 (23.4) | 76 (20.9) | 141 (23.7) | |
| $100 000–$150 000 | 84 (15.0) | 43 (9.7) | 11 (12.1) | 114 (13.0) | 36 (14.3) | 91 (12.5) | 37 (10.2) | 84 (14.1) | |
| More than $150 000 | 66 (11.8) | 61 (13.7) | 7 (7.7) | 119 (13.5) | 25 (9.9) | 102 (14.0) | 43 (11.8) | 81 (13.6) | |
| Refuse | 91 (16.3) | 79 (17.8) | 19 (20.9) | 141 (16.0) | 44 (17.5) | 120 (16.5) | 65 (17.9) | 92 (15.5) | |
| Not sure | 51 (9.1) | 52 (11.7) | 16 (17.6) | 82 (9.3) | 31 (12.3) | 67 (9.2) | 49 (13.5) | 49 (8.2) | |
| Race/ethnicity | |||||||||
| Non-Hispanic White | 250 (44.7) | 190 (42.8) | 30 (33.0) | 396 (45.0) | 105 (41.7) | 325 (44.6) | 144 (39.7) | 274 (46.1) | |
| Non-Hispanic Black | 27 (4.8) | 18 (4.1) | 2 (2.2) | 43 (4.9) | 9 (3.6) | 35 (4.8) | 15 (4.1) | 29 (4.9) | |
| Hispanic (any race) | 125 (22.4) | 127 (28.6) | 36 (39.6) | 209 (23.8) | 65 (25.8) | 182 (25.0) | 104 (28.7) | 141 (23.7) | |
| Asian | 46 (8.2) | 37 (8.3) | 3 (3.3) | 75 (8.5) | 20 (7.9) | 60 (8.2) | 38 (10.5) | 40 (6.7) | |
| Native American | 10 (1.8) | 7 (1.6) | 0 (0.0) | 17 (1.9) | 4 (1.6) | 13 (1.8) | 5 (1.4) | 12 (2.0) | |
| Native Hawaiian | 4 (0.7) | 1 (0.2) | 1 (1.1) | 4 (0.5) | 2 (0.8) | 3 (0.4) | 4 (1.1) | 1 (0.2) | |
| More than 1 race | 70 (12.5) | 51 (11.5) | 12 (13.2) | 107 (12.2) | 38 (15.1) | 83 (11.4) | 42 (11.6) | 74 (12.5) | |
| Refuse | 27 (4.8) | 13 (2.9) | 7 (7.7) | 29 (3.3) | 9 (3.6) | 27 (3.7) | 11 (3.0) | 23 (3.9) | |
| Region | |||||||||
| Predominantly urban regions | |||||||||
| San Francisco Bay area | 52 (9.3) | 55 (12.4) | 9 (9.9) | 96 (10.9) | 17 (6.7) | 89 (12.2) | 36 (9.9) | 66 (11.1) | |
| Greater Los Angeles area | 47 (8.4) | 49 (11.0) | 13 (14.3) | 82 (9.3) | 19 (7.5) | 78 (10.7) | 33 (9.1) | 64 (10.8) | |
| Greater Sacramento area | 64 (11.4) | 64 (14.4) | 15 (16.5) | 106 (12.0) | 36 (14.3) | 84 (11.5) | 52 (14.3) | 69 (11.6) | |
| San Diego and southern border | 59 (10.6) | 37 (8.3) | 8 (8.8) | 85 (9.7) | 24 (9.5) | 70 (9.6) | 35 (9.6) | 56 (9.4) | |
| Predominantly rural regions | |||||||||
| Central Coast | 74 (13.2) | 53 (11.9) | 11 (12.1) | 115 (13.1) | 28 (11.1) | 97 (13.3) | 49 (13.5) | 74 (12.5) | |
| Northern Sacramento Valley | 58 (10.4) | 48 (10.8) | 10 (11.0) | 93 (10.6) | 33 (13.1) | 70 (9.6) | 29 (8.0) | 73 (12.3) | |
| San Joaquin Valley | 65 (11.6) | 46 (10.4) | 6 (6.6) | 102 (11.6) | 34 (13.5) | 76 (10.4) | 44 (12.1) | 61 (10.3) | |
| Northwestern California | 72 (12.9) | 44 (9.9) | 12 (13.2) | 99 (11.2) | 38 (15.1) | 74 (10.2) | 47 (12.9) | 62 (10.4) | |
| Sierras region | 68 (12.2) | 48 (10.8) | 7 (7.7) | 102 (11.6) | 23 (9.1) | 90 (12.4) | 38 (10.5) | 69 (11.6) | |
| County reopening tier | |||||||||
| Purple (most restrictive) | 102 (18.2) | 98 (22.1) | 23 (25.3) | 174 (19.8) | 59 (23.4) | 140 (19.2) | 84 (23.1) | 112 (18.9) | |
| Red | 113 (20.2) | 92 (20.7) | 19 (20.9) | 183 (20.8) | 56 (22.2) | 145 (19.9) | 80 (22.0) | 119 (20.0) | |
| Orange | 97 (17.4) | 103 (23.2) | 13 (14.3) | 183 (20.8) | 43 (17.1) | 154 (21.2) | 72 (19.8) | 122 (20.5) | |
| Yellow (least restrictive) | 13 (2.3) | 10 (2.3) | 1 (1.1) | 22 (2.5) | 6 (2.4) | 17 (2.3) | 9 (2.5) | 14 (2.4) | |
| After 15 June | 234 (41.9) | 141 (31.8) | 35 (38.5) | 318 (36.1) | 88 (34.9) | 272 (37.4) | 118 (32.5) | 227 (38.2) | |
| Vaccination | |||||||||
| Unvaccinated | 326 (63.3) | 321 (74.5) | 53 (61.6) | 578 (69.6) | 136 (58.1) | 496 (71.8) | 230 (68.2) | 393 (69.3) | |
| Partially vaccinateda | 45 (8.7) | 38 (8.8) | 9 (10.5) | 71 (8.6) | 37 (15.8) | 45 (6.5) | 37 (11.0) | 43 (7.6) | |
| Fully vaccinated | 144 (28.0) | 72 (16.7) | 24 (27.9) | 181 (21.8) | 61 (26.1) | 150 (21.7) | 70 (20.8) | 131 (23.1) | |
| Symptoms experienced | |||||||||
| No symptoms | 246 (44.0) | 160 (36.0) | 50 (54.9) | 336 (38.2) | 128 (50.8) | 263 (36.1) | 166 (45.7) | 214 (36.0) | |
| Symptoms | 313 (56.0) | 284 (64.0) | 41 (45.1) | 544 (61.8) | 124 (49.2) | 465 (63.9) | 197 (54.3) | 380 (64.0) | |
| Level of anxiety about coronavirus disease 2019 | |||||||||
| Very anxious | 89 (15.9) | 66 (14.9) | 17 (18.7) | 135 (15.3) | 40 (15.9) | 114 (15.7) | 57 (15.7) | 93 (15.7) | |
| Not very anxious | 470 (84.1) | 378 (85.1) | 74 (81.3) | 745 (84.7) | 212 (84.1) | 614 (84.3) | 306 (84.3) | 501 (84.3) | |
Percentages presented in the table should be interpreted to represent the proportion of cases or controls with each characteristic and not their prevalence within the general population.
An individual was considered partially vaccinated if their severe acute respiratory syndrome coronavirus 2 test date with <14 days before their second dose of an mRNA vaccine product (Pfizer/BioNTech BNT-162b2 or Moderna mRNA-1273) or <14 days after their first dose of a single-dose product (Jansen Pharmaceutical Companies JNJ-78436735).
The majority (816 of 1006; 81%) of participants reporting high-risk exposure indicated that both they and their contact did not wear a mask during the interaction (Table 3). Mask usage did not differ substantially among participants by age, region, income strata, or in association with vaccination status. However, a higher proportion of individuals who reported unmasked interactions were non-Hispanic Whites (382/816; 47%) in comparison with participants reporting masked interactions (59 of 188; 31%). Most enrolled participants were unvaccinated (649 of 1006; 65%) at the time of testing; 8% (83 of 1006) and 22% (217 of 1006) were partially or fully vaccinated, respectively. Vaccination coverage varied over the study period, reflecting the continuous rollout of vaccination over time.
Table 3.
Distribution of Exposures Among Respondents Reporting Differing Types of Recent Contact With an Individual Known or Suspected to Have Severe Acute Respiratory Syndrome Coronavirus 2 Infection
| Participant Attribute | Mask Usage | Vaccinationa | ||||
|---|---|---|---|---|---|---|
| No Masks Worn | Mask Used by Participant or Contact | Unvaccinated | Partially Vaccinated | Fully Vaccinated | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
| N = 816 | N = 188 | N = 649 | N = 83 | N = 217 | ||
| Age, years | ||||||
| 0–6 | 34 (4.2) | 3 (1.6) | 37 (5.7) | 0 (0.0) | 0 (0.0) | |
| 7–12 | 46 (5.6) | 15 (8.0) | 60 (9.2) | 0 (0.0) | 0 (0.0) | |
| 13–17 | 51 (6.2) | 15 (8.0) | 53 (8.2) | 1 (1.2) | 8 (3.7) | |
| 18–29 | 250 (30.6) | 49 (26.1) | 201 (31.0) | 35 (42.2) | 49 (22.6) | |
| 30–49 | 286 (35.0) | 65 (34.6) | 203 (31.3) | 27 (32.5) | 100 (46.1) | |
| 50–64 | 105 (12.9) | 31 (16.5) | 72 (11.1) | 13 (15.7) | 39 (18.0) | |
| ≥65 | 44 (5.4) | 10 (5.3) | 23 (3.5) | 7 (8.4) | 21 (9.7) | |
| Sex | ||||||
| Male | 365 (44.7) | 94 (50.0) | 315 (48.5) | 39 (47.0) | 82 (37.8) | |
| Female | 451 (55.3) | 94 (50.0) | 334 (51.5) | 44 (53.0) | 135 (62.2) | |
| Household income | ||||||
| Less than $50 000 | 203 (24.9) | 42 (22.3) | 171 (26.3) | 18 (21.7) | 42 (19.4) | |
| $50 000–$100 000 | 191 (23.4) | 41 (21.8) | 158 (24.3) | 15 (18.1) | 48 (22.1) | |
| $100 000–$150 000 | 102 (12.5) | 25 (13.3) | 57 (8.8) | 14 (16.9) | 46 (21.2) | |
| More than $150 000 | 112 (13.7) | 16 (8.5) | 66 (10.2) | 8 (9.6) | 44 (20.3) | |
| Refuse | 132 (16.2) | 36 (19.1) | 127 (19.6) | 16 (19.3) | 23 (10.6) | |
| Not sure | 76 (9.3) | 28 (14.9) | 70 (10.8) | 12 (14.5) | 14 (6.5) | |
| Race/ethnicity | ||||||
| Non-Hispanic White | 382 (46.8) | 59 (31.4) | 255 (39.3) | 40 (48.2) | 122 (56.2) | |
| Non-Hispanic Black | 37 (4.5) | 8 (4.3) | 35 (5.4) | 1 (1.2) | 6 (2.8) | |
| Hispanic (any race) | 194 (23.8) | 58 (30.9) | 177 (27.3) | 25 (30.1) | 39 (18.0) | |
| Asian | 61 (7.5) | 22 (11.7) | 46 (7.1) | 7 (8.4) | 22 (10.1) | |
| Native American | 17 (2.1) | 0 (0.0) | 15 (2.3) | 0 (0.0) | 2 (0.9) | |
| Native Hawaiian | 2 (0.2) | 3 (1.6) | 5 (0.8) | 0 (0.0) | 0 (0.0) | |
| More than 1 race | 95 (11.6) | 27 (14.4) | 86 (13.3) | 9 (10.8) | 19 (8.8) | |
| Refuse | 28 (3.4) | 11 (5.9) | 30 (4.6) | 1 (1.2) | 7 (3.2) | |
| Region | ||||||
| Predominantly urban regions | ||||||
| San Francisco Bay area | 87 (10.7) | 21 (11.2) | 67 (10.3) | 8 (9.6) | 22 (10.1) | |
| Greater Los Angeles area | 86 (10.5) | 12 (6.4) | 68 (10.5) | 5 (6.0) | 21 (9.7) | |
| Greater Sacramento area | 92 (11.3) | 36 (19.1) | 90 (13.9) | 11 (13.3) | 20 (9.2) | |
| San Diego and southern Border | 81 (9.9) | 15 (8.0) | 60 (9.2) | 8 (9.6) | 24 (11.1) | |
| Predominantly rural regions | ||||||
| Central Coast | 106 (13.0) | 21 (11.2) | 74 (11.4) | 13 (15.7) | 33 (15.2) | |
| Northern Sacramento Valley | 90 (11.0) | 15 (8.0) | 68 (10.5) | 5 (6.0) | 28 (12.9) | |
| San Joaquin Valley | 88 (10.8) | 23 (12.2) | 65 (10.0) | 16 (19.3) | 21 (9.7) | |
| Northwestern California | 94 (11.5) | 22 (11.7) | 73 (11.2) | 7 (8.4) | 31 (14.3) | |
| Sierras region | 92 (11.3) | 23 (12.2) | 84 (12.9) | 10 (12.0) | 17 (7.8) | |
| County reopening tier | ||||||
| Purple (most restrictive) | 154 (18.9) | 47 (25.0) | 181 (27.9) | 14 (16.9) | 3 (1.4) | |
| Red | 167 (20.5) | 38 (20.2) | 145 (22.3) | 34 (41.0) | 25 (11.5) | |
| Orange | 177 (21.7) | 22 (11.7) | 150 (23.1) | 17 (20.5) | 27 (12.4) | |
| Yellow (least restrictive) | 22 (2.7) | 1 (0.5) | 14 (2.2) | 2 (2.4) | 4 (1.8) | |
| After 15 June | 296 (36.3) | 80 (42.6) | 159 (24.5) | 16 (19.3) | 158 (72.8) | |
| Vaccination | ||||||
| Unvaccinated | 539 (69.5) | 108 (62.8) | – – | – – | – – | |
| Partially vaccinated | 64 (8.3) | 19 (11.0) | – – | – – | – – | |
| Fully vaccinated | 172 (22.2) | 45 (26.2) | – – | – – | – – | |
| Symptoms experienced | ||||||
| No symptoms | 304 (37.3) | 101 (53.7) | 232 (35.7) | 39 (47.0) | 99 (45.6) | |
| Symptoms | 512 (62.7) | 87 (46.3) | 417 (64.3) | 44 (53.0) | 118 (54.4) | |
| Level of anxiety about coronavirus disease 2019 | ||||||
| Very anxious | 122 (15.0) | 35 (18.6) | 100 (15.4) | 10 (12.0) | 37 (17.1) | |
| Not very anxious | 694 (85.0) | 153 (81.4) | 549 (84.6) | 73 (88.0) | 180 (82.9) | |
Percentages presented in the table should be interpreted to represent the proportion of cases or controls with each characteristic and not their prevalence within the general population.
An individual was considered partially vaccinated if their severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) test date was <14 days before their second dose of an mRNA vaccine product (Pfizer/BioNTech BNT-162b2 or Moderna mRNA-1273) or <14 days after their first dose of a single-dose vaccine product (Jansen Pharmaceutical Companies JNJ-78436735). An individual was considered fully vaccinated if their SARS-CoV-2 test date was >14 days after their second dose of an mRNA vaccine product (BNT-162b2 or mRNA-1273) or >14 days after their first dose of a single-dose product (JNJ-78436735).
Predictors of Infection
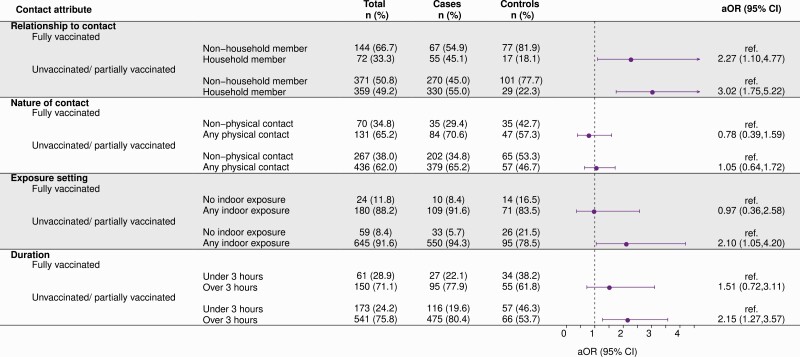
Among unvaccinated or partially vaccinated participants, cases were more likely to report high-risk exposures involving a potentially infected household member, occurring indoors, lasting ≥3 hours, or where either they or their contact did not wear a face mask (Figure 1, Supplementary Table 6). Adjusted odds of contact having occurred indoors, having lasted ≥3 hours, and having occurred with a household member were 2.10-fold (95% CI, 1.05–4.20), 2.15-fold (95% CI, 1.27–3.57), and 3.02-fold (95% CI, 1.75–5.22) higher among cases than controls. In contrast, we did not identify an association between case status and whether participants reported physical contact with the individual known or suspected to being infected. The association of each of these exposures with case status was mitigated among fully vaccinated participants, as indicated by lower point estimates of the aOR for each exposure among fully vaccinated participants compared with other participants. Estimated aORs were similar in models restricted to participants who specified that their contact was confirmed to have been infected with SARS-CoV-2 at the time of their interaction and among individuals who indicated this exposure was a primary motivation for testing (Supplementary Tables 7 and 8, Supplementary Figure 1).
Figure 1.
Predictors of infection following high-risk exposure. aORs computed using conditional logistic regression models interacting vaccination status with each contact attribute and adjusting for community exposures (listed in the main text), vaccination status (defined as fully vaccinated or unvaccinated/incompletely vaccinated) of the participant, mask-wearing by the participant and their contact, level of anxiety about coronavirus disease 2019 prior to testing, and participants’ age, sex, and region of residence. Regression strata were defined for county reopening tiers and, for the period after 15 June, the month of severe acute respiratory syndrome coronavirus 2 test. Further regression parameter estimates are presented in Supplementary Table 4. Counts for cases and controls differ from those listed in Table 1 because some participants indicated that they did not know these details about their known or suspected contact and because of missing data on vaccination status among cases (N = 8) and controls (N = 18). Abbreviations: aOR, adjusted odds ratio; CI, confidence interval.
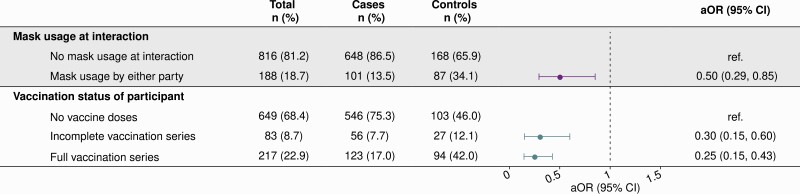
Among study participants, 14% of cases (101 of 749) and 34% of controls (87 of 255) reported mask usage during the high-risk interaction (aOR = 0.50; 95% CI, .29–.85; Figure 2). Estimated effect size estimates did not differ appreciably according to whether masks were worn exclusively by participants or their contacts, although analyses were underpowered to demonstrate significant effects within each of these strata or to make comparisons across them (Supplementary Figure 2). However, mask usage was protective when both parties reported mask usage during the interaction (aOR = 0.50; 95% CI, .26–.96). Adjusted odds of cases status were lower for both partially (aOR = 0.30; 95% CI, .16–.60) and fully vaccinated (aOR = 0.25; 95% CI, .15–.43) participants relative to unvaccinated participants.
Figure 2.
Protective effects of mask-wearing and vaccination in the context of high-risk exposure. aORs computed using conditional logistic regression models adjusting for vaccination status, community exposures (listed in the main text), characteristics of high-risk contact, level of anxiety about coronavirus disease 2019 prior to testing, and participants’ age, sex, and region of residence. Regression strata were defined for county reopening tiers and week of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) test. An individual was considered fully vaccinated if their SARS-CoV-2 test date was >14 days after their second dose of an mRNA vaccine product (Pfizer/BioNTech BNT-162b2 or Moderna mRNA-1273) or >14 days after their first dose of a single-dose product (Jansen Pharmaceutical Companies JNJ-78436735). In sensitivity analyses limiting to those who received an mRNA vaccine product (excluding N = 25 recipients of JNJ-78436735), the aORs (95% CIs) for incompletely vaccinated and fully vaccinated individuals were 0.30 (.14–.63) and 0.26 (.14–.46), respectively. Abbreviations: aOR, adjusted odds ratio; CI, confidence interval.
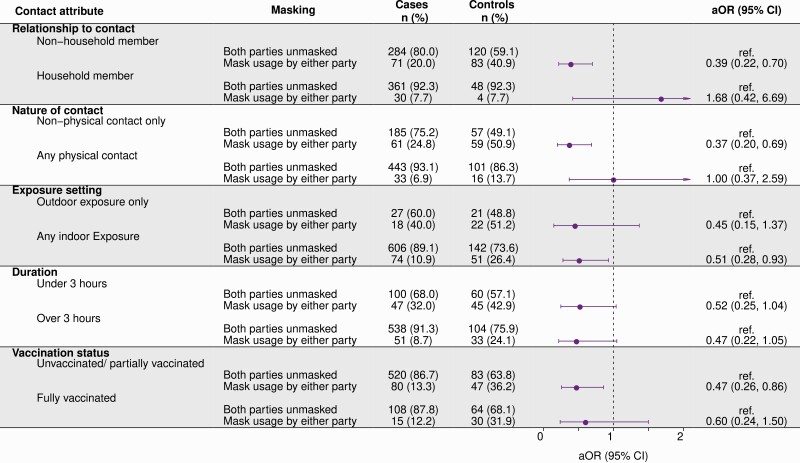
Protective effects of mask usage by either participants or their contacts differed according to several characteristics of exposure events. Mask usage was protective among participants reporting exposures to infected individuals outside their household (aOR = 0.39; 95% CI, .22–.70), exposures that occurred without physical contact (aOR = 0.37; 95% CI, .20–.69), and indoor exposures (aOR = 0.51; 95% CI, .28–.93; Figure 3). In contrast, we did not identify significant protective effects of mask usage when exposure involved an infected household member, involved physical contact, or occurred outdoors. Among unvaccinated or partially vaccinated participants; 13% of cases (80 of 600) and 36% of controls (47 of 130) reported mask usage during the interaction; and adjusted odds of mask usage were 0.47-fold (95% CI, .26–.86) as high among cases compared with controls. Among fully vaccinated participants, 12% of cases (15 of 123) and 32% of controls (30 of 94) reported mask usage during the interaction, and adjusted odds of mask usage were 0.60-fold (95% CI, .24–1.50) as high among cases compared with controls.
Figure 3.
Protective effects of mask-wearing in differing high-risk exposure contexts. aORs computed using conditional logistic regression models adjusting for vaccination status of respondent, community exposures (listed in main text), characteristics of the high-risk contact, level of coronavirus disease 2019 anxiety prior to testing, and participants’ age, sex, and region of residence. An interaction term was included between mask usage and the contact attribute in 5 separate models. Regression strata were defined for county reopening tiers and week of severe acute respiratory syndrome coronavirus 2 test. The aOR represents the aOR for case status comparing mask usage within each category (with respect to relationship, physical/nonphysical nature of contact, indoor/outdoor exposure, duration, and participant vaccination status). Abbreviations: aOR, adjusted odds ratio; CI, confidence interval.
Among 751 cases testing positive for SARS-CoV-2 who reported high-risk exposures, 187 (25%) indicated receiving healthcare beyond testing alone (Table 4). The aOR for mask usage during high-risk interactions was 0.69 (0.36–1.34) for cases who sought care with a medical provider compared with cases who did not. The proportion of cases experiencing symptoms likewise did not vary according to whether high-risk exposures involved a household member, involved physical contact, occurred indoors or outdoors, or lasted <3 hours or ≥3 hours. Similarly, neither the likelihood of experiencing symptoms nor the number of symptoms that participants reported experiencing differed according to these exposure attributes (Supplementary Tables 9–11).
Table 4.
Comparison of Infection Severity Among Cases Who Reported High-Risk Exposures With and Without Mask Usage
| Contact Attribute | Level of Care Sought | ||
|---|---|---|---|
| No Care Sought, n (%) | Care Sought, n (%) | Adjusted Odds Ratio (95% Confidence Interval) | |
| N = 564 | N = 187 | ||
| Mask usage at interaction | |||
| No mask usage at interaction | 482 (85.8) | 166 (88.8) | ref. |
| Mask usage by either party | 80 (14.2) | 21 (11.2) | 0.70 (.36–1.34) |
| Relationship to contact | |||
| Nonhousehold member | 264 (46.9) | 92 (49.7) | ref. |
| Household member | 299 (53.1) | 93 (50.3) | 0.97 (.62–1.50) |
| Nature of contact | |||
| Nonphysical contact only | 189 (34.7) | 58 (32.2) | ref. |
| Any physical contact | 355 (65.3) | 122 (67.8) | 0.89 (.56–1.41) |
| Setting | |||
| No indoor exposure | 34 (6.2) | 13 (7.1) | ref. |
| Any indoor exposure | 515 (93.8) | 171 (92.9) | 0.48 (.21–1.08) |
| Duration, hours | |||
| <3 | 114 (20.6) | 33 (17.9) | ref. |
| >3 | 440 (79.4) | 151 (82.1) | 1.18 (.66–2.14) |
Adjusted odds ratio (aOR) computed using logistic regression models restricted to cases who reported high-risk contact. Differences between total values (N) in the top row and sums of participants according to each exposure characteristic reflect missing data. Models adjusted for vaccination status of respondent, community exposures (listed in main text), characteristics of the high-risk exposure (as listed in Figure 1), and participants’ age, sex, and region of residence. The aOR represents the aOR for healthcare receipt among cases, according to the indicated attributes of high-risk exposure events reported by participants. We present the mean number of symptoms experienced among cases stratified by mask usage in Supplementary Table 9 during the high-risk exposure. Supplementary Table 10 indicates the presence of symptoms and mean number of symptoms, respectively, according to other attributes of the high-risk exposure. Supplementary Table 11 presents aORs for symptoms as an alternative measure of severity. Percentages presented in the table should be interpreted to represent the proportion of cases or controls with each characteristic and not their prevalence within the general population.
DISCUSSION
Among participants in our study who reported recent high-risk exposures, use of face masks was associated with reduced odds of testing positive for SARS-CoV-2 infection. Interacting in an indoor setting, longer (≥3 hour) lengths of interaction, and exposures involving household members were each associated with increased odds of testing positive for SARS-CoV-2 infection among participants who were not fully vaccinated. Among fully vaccinated participants, excess infection risk associated with exposure characteristics including unmasked contact, indoor contact, physical contact, and contact with a household member was mitigated. While associations between risk-reducing behaviors and test-seeking may be of concern in test-negative design studies of SARS-CoV-2 infection, our analyses controlled for participants’ self-reported levels of concern about the COVID-19 pandemic, and our findings held in sensitivity analyses restricted to individuals who reported that concerns about their high-risk exposure were a primary reason for test-seeking. These findings may inform the use of NPIs in populations with limited vaccine access or those ineligible to be vaccinated, as well as in response to changing epidemiologic conditions such as emergence of variants associated with enhanced infectiousness.
Whereas mask usage was protective in interactions where participants reported no physical contact with a potentially infectious individual, we did not identify protection in interactions where physical contact was made. Mask usage was also less clearly protective when participants were exposed to a potentially infected member of their own household. This finding may reflect the difficulty of adhering to stringent masking over periods of extended or repeated exposure, as may occur among household members [28, 29]. Our analysis provided the strongest evidence of benefits of masking for unvaccinated participants, although we also estimated 40% lower odds of infection associated with mask wearing among fully vaccinated participants. While this estimate did not exclude the possibility of no effect, analyses within the fully vaccinated stratum were underpowered due to the low numbers of participants experiencing post-vaccination infections.
Contrary to prevailing hypotheses [24], we did not identify strong evidence of associations between measures of infection severity and the likelihood for cases to report unmasked, indoor, long-lasting, or physical interactions with their potentially infected contacts. While bias may have occurred if individuals’ decision to wear masks was associated with their likelihood of seeking testing when asymptomatic or minimally symptomatic, receipt of care in a clinical setting provides a more objective indication of infection severity. Direct measurement of SARS-CoV-2 exposure intensity and clinical status was not possible under this design. However, based on our observations, real-world effects of masking and other nonpharmaceutical mitigation measures may have greater impact on individuals’ risk of infection than their likelihood of experiencing symptoms, once infected. Studies in animal models have likewise provided inconsistent support for the hypothesis that reducing SARS-CoV-2 exposure dose may lower the risk of severe disease, given infection [26].
While the test-negative design that we used in this analysis has historically been used primarily for studies of pathogen-specific interventions such as vaccines [30, 31], several features of our study design make this design applicable for NPIs, despite their potential for effects on multiple respiratory pathogens. Restricting our analytic sample to individuals who came into contact with COVID-19 cases during the 14 days before testing supports our effort to assess how features of known or suspected SARS-CoV-2 exposure events affect transmission of SARS-CoV-2 specifically. Furthermore, because transmission of respiratory pathogens other than SARS-CoV-2 has remained at historically low levels in California and much of the United States throughout the COVID-19 pandemic [32, 33], the likelihood for other infections to cause test-seeking within our study population is low.
Additional factors that may have modified the likelihood of transmission during high-risk exposure could include the vaccination status of infected contacts [34], the type of masks or face coverings used [35], the physical distance individuals maintained while interacting, and ventilation of indoor spaces where interactions occurred. Obtaining reliable information on these details of each interaction was not feasible through retrospective interviews with participants. While our sample size was not powered to distinguish between protection associated with masking by participants, their contacts, or both parties, confounding may also arise if the decision to wear masks was influenced by factors we did not measure, including contacts’ vaccination status. This may bias effect size estimates from our study toward the null, along with several other factors including exposure misclassification resulting from our reliance on self-reported behaviors, imperfect knowledge of contacts’ infection status, and the possibility that participants were infected through interactions other than the high-risk exposure events analyzed.
Our findings provide real-world evidence that NPIs including mask usage reduce risk of SARS-CoV-2 transmission when infectious and susceptible individuals come into contact. We also demonstrate substantial vaccine effectiveness against SARS-CoV-2 in the context of high-risk interactions, suggesting such exposures are not associated with heightened risk of vaccine failure. Study participants were mainly enrolled prior to the Delta variant becoming the predominant SARS-CoV-2 lineage in California. Nonetheless, multiple observational studies have confirmed persistence of vaccine protection against SARS-CoV-2 infection despite the emergence and circulation of new variants [36] and high vaccine effectiveness against severe outcomes including hospitalization and death when post-vaccination infections occur [37]. Amid efforts to increase vaccine uptake as a primary public health strategy, our findings indicate NPIs can protect unvaccinated persons and may also be valuable for vaccinated persons as measures to reduce SARS-CoV-2 transmission.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Members of the California COVID-19 Case-Control Study Team include Helia Samani, Nikolina Walas, Erin Xavier, Diana J. Poindexter, Najla Dabbagh, Michelle M. Spinosa, Shrey Saretha, Adrian F. Cornejo, Hyemin Park, Miriam I. Bermejo, Amanda Lam, Amandeep Kaur, Ashly Dyke, Diana Felipe, Maya Spencer, Savannah Corredor, and Yasmine Abdulrahim.
Financial support. This work was supported by the California Department of Public Health. J. P., J. O., and J. F. M. were supported by grant 5-NU50CK000539 from the Epidemiology & Laboratory Capacity for Infectious Diseases Program of the US Centers for Disease Control and Prevention (program 0187.0150). J. A. L. was supported by grant R01-AI14812701 from the National Institute of Allergy and Infectious Diseases.
Potential conflicts of interest. J. A. L. discloses receipt of grant funding and consulting fees from Pfizer, Inc, Merck, Sharp & Dohme, and VaxCyte unrelated to this study. All remaining authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Kristin L Andrejko, Division of Epidemiology and Biostatistics, School of Public Health, University of California, Berkeley, California, USA.
Jake Pry, California Department of Public Health, Richmond, California, USA.
Jennifer F Myers, California Department of Public Health, Richmond, California, USA.
John Openshaw, California Department of Public Health, Richmond, California, USA.
James Watt, California Department of Public Health, Richmond, California, USA.
Nozomi Birkett, California Department of Public Health, Richmond, California, USA.
Jennifer L DeGuzman, California Department of Public Health, Richmond, California, USA.
Camilla M Barbaduomo, California Department of Public Health, Richmond, California, USA.
Zheng N Dong, California Department of Public Health, Richmond, California, USA.
Anna T Fang, California Department of Public Health, Richmond, California, USA.
Paulina M Frost, California Department of Public Health, Richmond, California, USA.
Timothy Ho, California Department of Public Health, Richmond, California, USA.
Mahsa H Javadi, California Department of Public Health, Richmond, California, USA.
Sophia S Li, California Department of Public Health, Richmond, California, USA.
Vivian H Tran, California Department of Public Health, Richmond, California, USA.
Christine Wan, California Department of Public Health, Richmond, California, USA.
Seema Jain, California Department of Public Health, Richmond, California, USA.
Joseph A Lewnard, Division of Epidemiology and Biostatistics, School of Public Health, University of California, Berkeley, California, USA; Division of Infectious Diseases & Vaccinology, School of Public Health, University of California, Berkeley, California, USA; Center for Computational Biology, College of Engineering, University of California, Berkeley, California, USA.
California COVID-19 Case-Control Study Team:
Helia Samani, Nikolina Walas, Erin Xavier, Diana J Poindexter, Najla Dabbagh, Michelle M Spinosa, Shrey Saretha, Adrian F Cornejo, Hyemin Park, Miriam I Bermejo, Amanda Lam, Amandeep Kaur, Ashly Dyke, Diana Felipe, Maya Spencer, Savannah Corredor, and Yasmine Abdulrahim
References
- 1. Andrejko KL, Pry J, Myers JF, et al. . Prevention of COVID-19 by mRNA-based vaccines within the general population of California. Clin Infect Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haas EJ, Angulo FJ, McLaughlin JM, et al. . Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021; 397:1819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dagan N, Barda N, Kepten E, et al. . BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. NEJM 2021; 384:1412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jones NR, Qureshi ZU, Temple RJ, Larwood JPJ, Greenhalgh T, Bourouiba L.. Two metres or one: what is the evidence for physical distancing in covid-19?. BMJ 2020; 370:m3223. [DOI] [PubMed] [Google Scholar]
- 5. Bulfone TC, Malekinejad M, Rutherford GW, Razani N.. Outdoor transmission of SARS-CoV-2 and other respiratory viruses: a systematic review. J Infect Dis 2021; 223:550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brooks JT, Butler JC.. Effectiveness of mask wearing to control community spread of SARS-CoV-2. JAMA 2021; 325:998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention. Science brief: community use of cloth masks to control the spread of SARS-CoV-2. 2020. [cited 2021 Jul 26]. Available at: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/masking-science-sars-cov2.html.
- 8. Semenza JC, Adlhoch C, Baka A, et al. . COVID-19 research priorities for non-pharmaceutical public health and social measures. Epidemiol Infect 2021; 149:e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCartney M. We need better evidence on non-drug interventions for COVID-19. BMJ 2020; 370:m3473. [DOI] [PubMed] [Google Scholar]
- 10. Greenhalgh T, Jimenez JL, Prather KA, Tufekci Z, Fisman D, Schooley R.. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet 2021; 397:1603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Y, Campbell H, Kulkarni D, et al. . The temporal association of introducing and lifting non-pharmaceutical interventions with the time-varying reproduction number (R) of SARS-CoV-2: a modelling study across 131 countries. Lancet Infect Dis 2021; 21:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lasry A, Kidder D, Hast M, et al. . Timing of community mitigation and changes in reported COVID-19 and community mobility―four U.S. metropolitan areas, February 26–April 1, 2020. MMWR 2020; 69:451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jarvis CI, Van Zandvoort K, Gimma A, et al. . Quantifying the impact of physical distance measures on the transmission of COVID-19 in the UK. BMC Med 2020; 18:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bauch CT. Estimating the COVID-19 R number: a bargain with the devil?. Lancet Infect Dis 2021; 21:151–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leung NHL, Chu DKW, Shiu EYC, et al. . Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med 2020; 26:676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Milton DK, Fabian MP, Cowling BJ, Grantham ML, McDevitt JJ.. Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog 2013; 9:e1003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cowling BJ, Chan, H, Fang VJ, et al. . Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med 2009; 151:437–46. [DOI] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention. Science brief: COVID-19 vaccines and vaccination. 2021. [cited 2021 Jul 26]. Available at: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/fully-vaccinated-people.html.
- 19. California Secretary of Health and Human Services. Safely reopening California. 2021. [cited 2021 Jul 22]. Available at: https://covid19.ca.gov/safely-reopening/.
- 20. State of California. Tracking COVID-19 in California—coronavirus COVID-19 response. 2021. [cited 2021 Jul 22]. Available at: https://Covid19CaGov.
- 21. Diesel J. COVID-19 vaccination coverage among adults—United States, December 14, 2020–May 22, 2021. MMWR 2021; 70:922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Public Health England. SARS-Cov-2 variant of concern and variants under investigation in England. 2021. [cited 2021 Jul 22]. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/986380/Variants_of_Concern_VOC_Technical_Briefing_11_England.pdf.
- 23. County of Los Angeles Public Health. County community transmission of COVID-19 increases from moderate to substantial; reinstating masking indoors for everyone. 2021. [cited 2021 Jul 22]. Available at: http://publichealth.lacounty.gov/phcommon/public/media/mediapubhpdetail.cfm?prid=3240.
- 24. Cheng Y, Ma N, Witt C, et al. . Face masks effectively limit the probability of SARS-CoV-2 transmission. Science 2021; 372:1439–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bielecki M, Züst R, Siegrist D, et al. . Social distancing alters the clinical course of COVID-19 in young adults: a comparative cohort study. Clin Infect Dis 2021; 72:598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spinelli MA, Glidden DV, Gennatas ED, et al. . Importance of non-pharmaceutical interventions in lowering the viral inoculum to reduce susceptibility to infection by SARS-CoV-2 and potentially disease severity. Lancet Infect Dis 2021; 21:e296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gandhi M, Rutherford GW.. Facial masking for COVID-19—potential for “variolation” as we await a vaccine. NEJM 2020; 383:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferng Y, Wong-McLoughlin J, Barrett A, Currie L, Larson E.. Barriers to mask wearing for influenza-like illnesses among urban Hispanic households. Public Health Nurs 2011; 28:13–23. [DOI] [PubMed] [Google Scholar]
- 29. Lee LY, Lam EP, Chan C, et al. . Practice and technique of using face mask amongst adults in the community: a cross-sectional descriptive study. BMC Public Health 2020; 20:948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lewnard JA, Tedijanto C, Cowling BJ, Lipsitch M.. Measurement of vaccine direct effects under the test-negative design. Am J Epidemiol 2018; 187:2686–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chua H, Feng S, Lewnard JA, et al. . The use of test-negative controls to monitor vaccine effectiveness: a systematic review of methodology. Epidemiology 2020; 31:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rizzo KR, Hoover C, Jain S, et al. . Influenza and SARS-CoV-2 co-infections in California, USA, September 2020–April 2021. Emerg Infect Dis 2021; 27:2923–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Centers for Disease Control and Prevention. Weekly U.S. influenza surveillance report. 2021. [cited 2021 Nov 29]. Available at: https://www.cdc.gov/flu/weekly/index.htm.
- 34. Levine-Tiefenbrun M, Yelin I, Katz R, et al. . Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med 2021; 27:790–2. [DOI] [PubMed] [Google Scholar]
- 35. Qaseem A, Etxeandia-Ikobaltzeta I, Yost J, et al. . Use of N95, surgical, and cloth masks to prevent COVID-19 in health care and community settings: living practice points from the American College of Physicians. Ann Intern Med 2020; 173:642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lopez Bernal J, Andrews N, Gower C, et al. . Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. NEJM 2021; 385:585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shapiro J, Dean NE, Madewell ZJ, Yang Y, Halloran ME, Longini I.. Efficacy estimates for various COVID-19 vaccines: what we know from the literature and reports. medRxiv 2021. doi: 10.1101/2021.05.20.21257461. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.