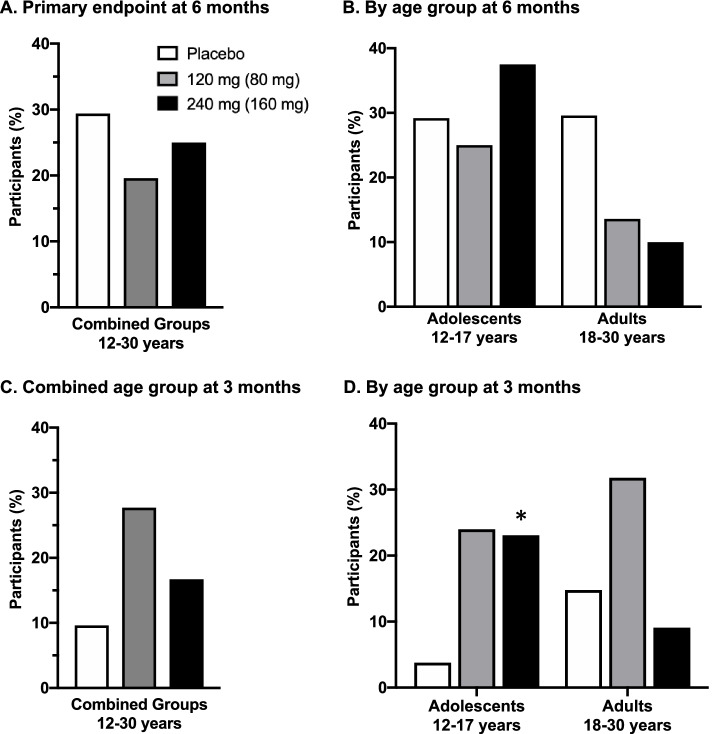
Fig. 2.
Percent of participants with above-threshold improvement on the composite endpoint. A Primary efficacy endpoint after 6 months of treatment. Percent of participants with above-threshold improvement: B by age group (adolescents, adults) after 6 months of treatment; C combined age group after 3 months of treatment; D by age group (adolescents, adults) after 3 months of treatment. Above-threshold improvement on the composite endpoint was defined as having (1) a relevant increase in raw scores from baseline in at least two out of three tasks from the Repeatable Battery for the Assessment of Neuropsychological Status ([RBANS]; ≥ 2 points for list learning, ≥ 1 point for list recognition, ≥ 1 point for list recall); and (2) either an increase from baseline in the Vineland Adaptive Behavior Scales-II (VABS II) composite score of ≥ 7 or a Down syndrome-specific Clinical Global Impression-Improvement (DS-CGI-I) ≤ 3 (minimally improved). Efficacy assessments were performed at baseline and after 3 and 6 months of treatment. Statistics: *p < 0.05 vs. placebo-treated group