Abstract
Background:
Colonoscopy is frequently performed in older adults, yet data on current use, and clinical outcomes of and follow-up recommendations after colonoscopy in older adults are lacking.
Methods:
This was an observational study using the New Hampshire Colonoscopy Registry of adults age ≥65 years undergoing colonoscopy for screening, surveillance of prior polyps, or evaluation of symptoms. The main outcomes were clinical findings of polyps and colorectal cancer and recommendations for future colonoscopy by age.
Results:
Between 2009 and 2019, there were 42,611 colonoscopies, of which 17,527 (41%) were screening, 19,025 (45%) surveillance, and 6059 (14%) for the evaluation of symptoms. Mean age was 71.1 years (SD 5.0), and 49.3% were male. The finding of colorectal cancer was rare (0.71%), with the highest incidence among diagnostic examinations (2.4%). The incidence of advanced polyps increased with patient age from 65–69 to ≥85 years for screening (7.1% to 13.6%; p = 0.05) and surveillance (9.4% to 12.0%; p < 0.001). Recommendations for future colonoscopy decreased with age and varied by findings at current colonoscopy. In patients without any significant findings, 85% aged 70–74 years, 61.9% aged 75–79 years, 39.1% aged 80–84 years, and 27.4% aged ≥85 years (p < 0.001) were told to continue colonoscopy. Among patients with advanced polyps, 97.2% aged 70–74 years, 89.6% aged 75–79 years, 78.4% aged 80–84 years, and 66.7% aged ≥85 years were told to continue colonoscopy (p < 0.001).
Conclusions:
Within this comprehensive statewide registry, clinical findings during colonoscopy varied by indication and increased with age. Overall rates of finding advanced polyps and colorectal cancer are low. Older adults are frequently recommended to continue colonoscopy despite advanced age and insignificant clinical findings on current examination. These data inform the potential benefits of ongoing colonoscopy, which must be weighed with the low but known potential immediate and long-term harms of colonoscopy, including cost, psychological distress, and long lag time to benefit exceeding life expectancy.
Keywords: age, colorectal cancer screening, polyp surveillance, recommendations
INTRODUCTION
Colonoscopy is frequently performed in older adults for colorectal cancer screening, surveillance after adenomatous polyps are detected, and diagnostic purposes to evaluate symptoms.1,2 However, there are few data on the current use and outcomes of colonoscopy in older adults. Colonoscopy literature in older adults has focused mostly on safety, demonstrating that older adults have a higher incidence of adverse events with colonoscopy compared to younger cohorts, regardless of the indication for colonoscopy.3–6 Prior studies evaluating clinical findings or follow-up recommendations after colonoscopy in older adults have been limited in their ability to account for granular patient- or procedure-level details, including colorectal cancer (CRC) risk factors such as family history of CRC and colonoscopy indication.7,8 Studies of the use and outcomes of colonoscopy in older adults are needed to understand the implications of current practice and help inform decision-making for older adults and their clinicians considering colonoscopy.
The New Hampshire Colonoscopy Registry (NHCR), a statewide colonoscopy registry provides a unique and valuable resource for the evaluation of population-based colonoscopy practice.9–11 Analyses using NHCR data have addressed many aspects of colonoscopy, including lifestyle factors associated with polyps,12 adenoma and serrated polyp detection rates,13,14 bowel preparation quality,15,16 and outcomes in young adults.10 However, NHCR studies focusing on older adults have been limited to a screening population without polyps.11 Our goal is to provide a more comprehensive evaluation of colonoscopy use and outcomes across all indications (screening, surveillance, diagnostic) in older adults. Low rates of clinically significant findings in older adults attending routine colonoscopy for screening or surveillance purposes could indicate overuse of colonoscopy, meriting improved assessment of patient age, risk factors, and previous colonoscopy findings in making decisions about whether to pursue colonoscopy.
METHODS
Study design
This was an observational study based on data of adults aged ≥65 years undergoing colonoscopy from the New Hampshire Colonoscopy Registry (NHCR).
The New Hampshire Colonoscopy Registry
The NHCR is a comprehensive, statewide registry founded in 2004 that collects and analyzes data from endoscopy sites throughout New Hampshire. It has been described in detail previously.9–11 Prior to colonoscopy, patients complete a self-administered questionnaire on demographic factors (e.g., age, sex, and education), health behaviors (e.g., smoking, alcohol intake, and exercise), personal history of polyps and CRC, and family history of CRC. The endoscopist or the endoscopy nurse completes the NHCR Colonoscopy Procedure Form during or immediately after colonoscopy. Data collected include a detailed indication for colonoscopy; findings (location, size, and specific treatment of polyps or cancer), type and quality of bowel preparation; sedation medication; anatomical location reached during the procedure; and follow-up recommendations. Examination indications are directly provided by the performing endoscopist and include screening, surveillance, and diagnostic categories. Polyp size is based on the endoscopist’s assessment and categorized as <5, 5–9, 10–20, or >20 mm. For all colonoscopies with findings, the NHCR requests pathology reports directly from the pathology laboratory used by each participating endoscopy facility. Trained NHCR staff abstract and enter these pathology findings into the NHCR database, including location, size, and histology of each polyp from the Colonoscopy Procedure Form. Outcomes regarding CRC are supplemented by linkage with NH State Cancer Registry.17
Inclusion criteria
We included all colonoscopies with completed patient questionnaires and procedure forms performed in adults age ≥65 years between April 2009, at which time the NHCR had expanded to include additional statewide sites, and October 2019.
Exclusion criteria
We excluded examinations in patients with a personal history of inflammatory bowel disease or genetic CRC syndromes (e.g., familial adenomatous polyposis or Lynch syndrome), due to the higher risk of CRC and need for more frequent colonoscopies than in the screening and surveillance population.
Outcomes
The primary outcomes were clinical findings during colonoscopy by age and indication. Secondary outcomes included recommendations for follow-up and colonoscopy metrics, including adequate bowel preparation and completion rates. Findings were categorized as not significant (no polyps or small hyperplastic polyps <10 mm), non-advanced polyps (one or more adenomas <10 mm without villous features or high-grade dysplasia or sessile serrated polyps <10 mm without dysplasia), or advanced polyps (defined as adenomas ≥10 mm in size, with high-grade dysplasia, or with villous features or sessile serrated polyps or hyperplastic polyps ≥10 mm or with dysplasia or traditional serrated adenoma) or CRC. The term advanced neoplasia was defined as advanced polyps or CRC. Indications were categorized as CRC screening (“screening”), surveillance in those with a personal history of colon polyps or CRC (“surveillance”), or for the evaluation of symptoms or abnormal imaging (“diagnostic”), corresponding to the indication categories listed on the NHCR Colonoscopy Procedure Form. Follow-up recommendation choices included follow-up at a recommended interval (≤1, 2–3, 4–5, 6–9, ≥10 years), recommendation pending pathology, no further colonoscopy indicated, or others (follow-up with PCP, other testing, etc.). Completion rates were defined as reaching the extent of the examination intended.
Patient, procedure, and endoscopist characteristics
Patient-level characteristics included age, sex, race, ethnicity, education, marital status, exercise, smoking history, alcohol intake, and self-reported general health (“how would you say your health is?”), personal history of CRC, and family history of a first-degree relative with CRC. Procedure-level characteristics included indication, practice setting, sedation used, completion status, reason for incomplete examinations where available (e.g., due to obstruction, sedation, tortuous colon, inadequate bowel preparation), bowel preparation quality (as assessed by standardized NHCR definitions applied on withdrawal of “excellent” “good” “fair” “poor”), and findings (as described above under “outcomes”).
The provision of a specific follow-up interval or “repeat with propofol” was considered a recommendation to repeat colonoscopy in the future. Conversely, a recommendation for “no further colonoscopy indicated” or “follow-up with primary care provider” alone was considered to stop colonoscopy. Recommendations of “follow-up recommendation pending pathology report” were excluded from the analysis of follow-up recommendations for future colonoscopy because of the inability to assess the final recommendation until after pathology was reviewed.
Endoscopist-level characteristics included sex, specialty (based on self-reported training), experience (years since completion of training), and adenoma detection rate (ADR). ADR was calculated among adults aged 50–75 years with screening colonoscopies that were complete to the cecum with an adequate bowel preparation, as the number of colonoscopies with one or more adenomas detected was divided by the total number of colonoscopies. ADR was calculated for endoscopists with at least 50 ADR-eligible colonoscopies in the NHCR database during the study timeframe.
Statistical analysis
The main analyses were at the event level, meaning that a single patient could contribute more than one colonoscopy to the dataset. For individuals with colonoscopies performed within 12 months of each other, findings from the second examination were merged with the first if the first colonoscopy was limited by inadequate preparation or was incomplete, or if the second colonoscopy was performed for the indication of treatment of a known polyp, as any findings on the second examination were unlikely to be de novo. A description of the characteristics of the cohort was performed at the patient level, such that for patients with multiple examinations, characteristics from their most recent examination were included.
We evaluated colonoscopies in adults aged ≥70 years at the time of colonoscopy in 5-year age groups (70–74, 75–79, 80–84, and ≥85) compared to the youngest cohort (age 65–69). We used descriptive statistics with means and standard deviations (SD) and medians and interquartile ranges (IQR) where appropriate. Initial comparisons of categorical data were made using Pearson’s chi-squared and Fisher’s exact tests. Initial comparisons of continuous data were made using one-way ANOVA or Kruskal–Wallis tests. The Cochran-Armitage test for trend was used to compare findings across age categories.
Proportional odds mixed models for ordinal outcomes with endoscopist random effects to account for clustering by endoscopist were used to provide adjusted estimates of the effect of patient and endoscopist characteristics on colonoscopy findings (categorized as no significant findings, non-advanced polyps, and advanced neoplasia due to few CRCs).18 Age, sex, examination indication, personal or family history of CRC, BMI, exercise, smoking, and alcohol use were included. Adjusted odds ratios and 95% confidence intervals (CIs) are presented. p-values <0.05 were considered statistically significant.
Human subjects’ protection
This study was approved by the Dartmouth-Hitchcock Institutional Review Board on October 24, 2018.
RESULTS
Between April 2009 and October 2019, there were 44,451 colonoscopies in patients aged ≥65 years, of which 42,611 met inclusion criteria; 17,527 (41%) screening, 19,025 (45%) surveillance, and 6059 (14%) diagnostic (Figure 1). These examinations were performed in 37,669 patients, of whom 4349 (11.5%) contributed multiple examinations.
FIGURE 1.
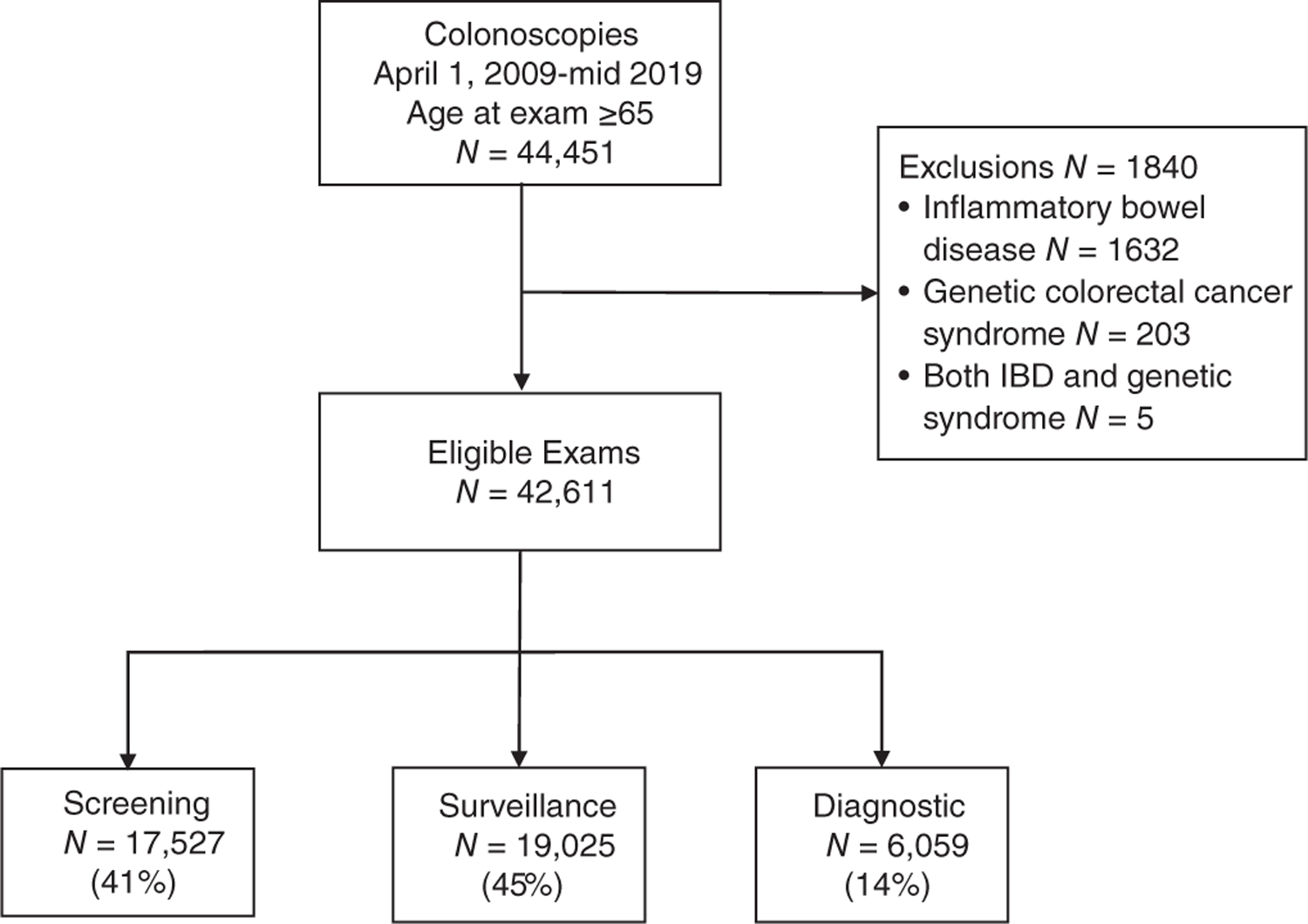
Flow diagram of colonoscopies among adults aged 65 years and older within the New Hampshire Colonoscopy Registry between 2009 and 2019 included in this study
Description of the cohort
Table 1 shows patient characteristics. Overall, mean age was 71.1 years (SD 5.0) and 49.3% were male. The cohort was mostly non-Hispanic Caucasian and 73.9% attained some college education or higher. Patients undergoing surveillance colonoscopy were more likely to be male and have a personal or family history of CRC. Patients undergoing screening colonoscopy were more likely to exercise regularly and have never smoked. Patients undergoing diagnostic colonoscopy tended to be older and report themselves as less healthy.
TABLE 1.
Characteristics of the 37,669 patients aged 65 years and older undergoing colonoscopy for surveillance, screening, or diagnostic purposes within the New Hampshire Colonoscopy Registry
| Indication of colonoscopy, N and % |
||||||
|---|---|---|---|---|---|---|
| Screening |
Surveillance |
Diagnostic |
||||
| Characteristic | N = 16,922 | N = 15,432 | N = 5315 | |||
| Age, years | ||||||
| 65–69 | 9578 | 56.6 | 7628 | 49.4 | 2077 | 39.1 |
| 70–74 | 4763 | 28.2 | 4347 | 28.2 | 1460 | 27.5 |
| 75–79 | 2013 | 11.9 | 2361 | 15.3 | 975 | 18.3 |
| 80–84 | 482 | 2.9 | 888 | 5.8 | 591 | 11.1 |
| ≥85 | 86 | 0.5 | 208 | 1.4 | 212 | 4.0 |
| Mean/SD | 70.31 | 4.4 | 71.31 | 5.0 | 73.01 | 6.0 |
| Median/IQR | 69.20 | 6.0 | 70.06 | 7.1 | 71.73 | 8.9 |
| Sexa | ||||||
| Male | 7706 | 45.7 | 8649 | 56.1 | 2182 | 41.2 |
| Female | 9165 | 54.3 | 6756 | 43.9 | 3121 | 58.9 |
| Racea | ||||||
| Caucasian | 15,557 | 97.4 | 13,638 | 97.1 | 4780 | 96.8 |
| Other | 419 | 2.6 | 412 | 2.9 | 156 | 3.2 |
| Hispanic ethnicitya | 211 | 1.4 | 167 | 1.3 | 60 | 1.3 |
| Martial statusa | ||||||
| Single | 880 | 5.5 | 707 | 5.0 | 268 | 5.4 |
| Married | 12,010 | 74.9 | 10,532 | 74.9 | 3362 | 68.3 |
| Previously married | 3151 | 19.6 | 2824 | 20.1 | 1296 | 26.3 |
| Educationa | ||||||
| Less than HS | 547 | 3.4 | 645 | 4.6 | 365 | 7.4 |
| HS/GED | 3151 | 19.6 | 3102 | 22.0 | 1355 | 27.5 |
| Some college/technical school | 3977 | 24.8 | 3490 | 24.8 | 1266 | 25.6 |
| College or more | 8376 | 52.2 | 6846 | 48.6 | 1951 | 39.5 |
| First colonoscopy | 1986 | 11.7 | 1 | 0.0 | 647 | 12.2 |
| Any personal history of CRC | 307 | 1.8 | 1803 | 11.7 | 263 | 5.0 |
| Family history of CRC in FDRa | 3883 | 23.0 | 4057 | 26.3 | 930 | 17.5 |
| Exercise | ||||||
| None | 1284 | 8.0 | 1392 | 9.9 | 814 | 16.6 |
| Active daily life | 5659 | 35.3 | 5267 | 37.5 | 1955 | 39.9 |
| 1–5 times per week | 7079 | 44.2 | 5869 | 41.8 | 1711 | 34.9 |
| 5+ times per week | 1994 | 12.5 | 1519 | 10.8 | 426 | 8.7 |
| Alcohol—drinks per weeka | ||||||
| None | 6403 | 40.0 | 5636 | 40.2 | 2513 | 51.4 |
| 1–4 | 4928 | 30.8 | 4115 | 29.4 | 1253 | 25.6 |
| 5–8 | 2743 | 17.1 | 2400 | 17.1 | 664 | 13.6 |
| 9–20 | 1753 | 11.0 | 1639 | 11.7 | 390 | 8.0 |
| 21 or more | 182 | 1.1 | 219 | 1.6 | 67 | 1.4 |
| Smoking statusa | ||||||
| Never smoked | 7750 | 48.4 | 5745 | 40.8 | 2006 | 40.7 |
| Former smoker | 7566 | 47.3 | 7570 | 53.8 | 2597 | 52.7 |
| Current smoker | 689 | 4.3 | 755 | 5.4 | 323 | 6.6 |
| General healtha | ||||||
| Good to excellent | 15,218 | 95.1 | 13,052 | 92.9 | 4106 | 83.6 |
| Fair | 740 | 4.6 | 902 | 6.4 | 702 | 14.3 |
| Poor | 52 | 0.3 | 89 | 0.6 | 103 | 2.1 |
Note: For the 4349 patients (11.5%) who contributed multiple examinations, the characteristics of their first examination were included here.
Missing data: Sex (90, 0.2%), race (2707, 7.2%), ethnicity (4411, 11.7%), marital status (2639, 7.0%), education (2598, 6.9%), family history (17, 0.05%), exercise (2700, 7.2%), smoking (2668, 7.1%), alcohol (2764, 7.3%), general health (2705, 7.2%).
Endoscopist characteristics
Colonoscopies were performed by 169 endoscopists, of whom 25.4% were female and 59.9% had >20-year experience. Overall, the median ADR (IQR) was 27.8 % (11.7). ADR was ≥30 for 43.6% of endoscopists, 20 to <30 for 36.3% of endoscopists, and <20 for 20.2% of endoscopists.
Colonoscopy characteristics
Adequate bowel preparation was achieved in 97.7% of examinations and 97.6% were complete to the cecum, although this was slightly lower in diagnostic examinations (95.3%, p < 0.001) (Table S1). The majority (57.5%) of examinations were performed using moderate sedation with 41.3% using monitored anesthesia care, which was slightly more frequent among diagnostic examinations (44.3%, p < 0.001). These examinations were performed in a mix of community hospitals (45.1%), ambulatory endoscopy centers (29.0%), and academic medical centers (25.1%).
Colonoscopy findings
Figure 2 shows colonoscopy findings by age for each category of examination indication. Overall, the finding of CRC was rare (0.71%), with a higher incidence among diagnostic (2.4%) compared to screening (0.47%) and surveillance (0.43%) examinations. For screening, the incidence of CRC increased by age: 0.25% (65–69 years) to 1.0% (80–84 years; p < 0.0001). CRC incidence also increased by age for surveillance (0.30% [65–69 years] to 1.7% [≥85 years; p = 0.001]) and diagnostic examinations (1.9% (65–69 years) to 8.0% (≥85 years; p < 0.0001)).
FIGURE 2.
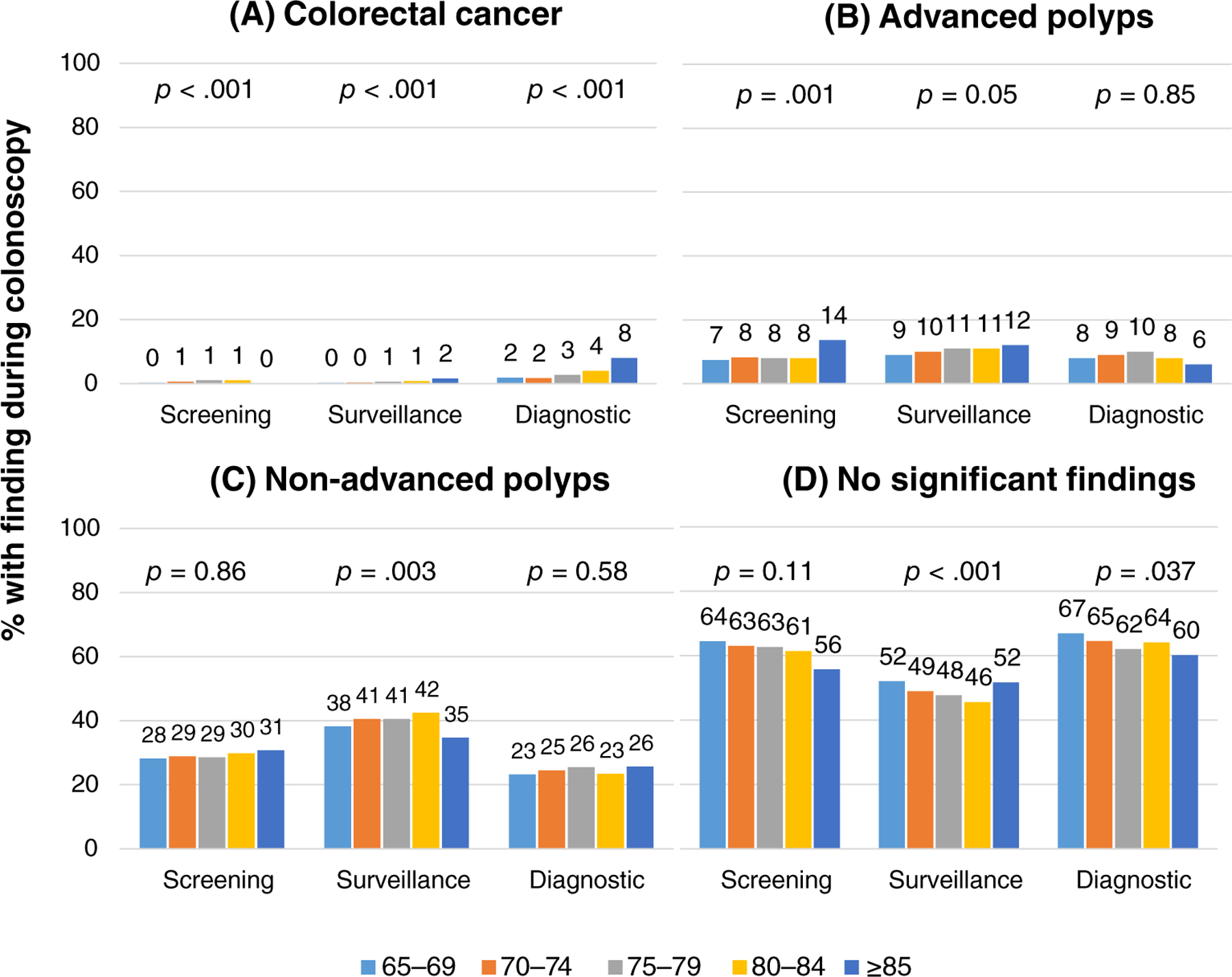
Most advanced finding during colonoscopy by patient age and indication. *Percentages have been rounded to the nearest whole number due to space constraints. (A) Colorectal cancer. (B) Advanced polyps. (C) Nonadvanced polyps. (D) No significant findings
For advanced polyps, there was an increase by age from 9.4% (65–69 years) to 12.0% (≥85 years; p = 0.029) among surveillance examinations. The incidence of advanced polyps ranged from 7.1% (65–69 years) to 13.6% (≥85 years; p = 0.14) among screening and 6.2% (65–69 years) to 9.7% (age 75–79; p = 0.27) among diagnostic examinations.
For each age group, the incidence of CRC was highest among patients presenting for diagnostic colonoscopy compared to screening and surveillance (Table S2).
Predictors of colonoscopy findings
Table 2 shows factors associated with the severity of detected neoplasia during colonoscopy in the model for ordered outcomes (no significant findings, non-advanced polyps, or advanced neoplasia) accounting for clustering by endoscopist. Advancing patient age, male sex, being overweight or obese, infrequent exercise, >5 alcoholic drinks/week, past or current smoking, and family history of CRC were associated with an increase in severity of detected neoplasia. Compared to screening, colonoscopy for surveillance was associated with greater odds of increased severity of findings (adjusted OR 1.38 [1.31–1.45], p < 0.0001).
TABLE 2.
Predictors of the most advanced findingsa on colonoscopy (N = 29,105) based on random effects model for ordinal data, accounting for clustering of data by endoscopist (N = 169)
| Variable | Odds ratiob | p |
|---|---|---|
| Age (5-year increments; ref = age 65–69) | 1.09 [1.07–1.12] | <0.0001 |
| Male (ref = female) | 1.62 [1.54–1.70] | <0.0001 |
| BMI (ref normal) | ||
| Underweight | 0.96 [0.80–1.15] | 0.65 |
| Overweight | 1.12 [1.05–1.19] | <0.0001 |
| Obese | 1.37 [1.28–1.46] | <0.0001 |
| Former or current smoker (ref = never smoker) | 1.16 [1.10–1.21] | <0.0001 |
| Alcohol intake (≥5 vs. <4 drinks/week) | 1.10 [1.04–1.16] | <0.0001 |
| Exercise (≥1 vs. <1 times/week) | 0.84 [0.80–0.88] | <0.0001 |
| History of colorectal cancer | 0.93 [0.84–1.03] | 0.18 |
| Family history of colorectal cancer in first-degree relative | 1.07 [1.01–1.13] | 0.022 |
| Indication (ref = screening) | ||
| Surveillance | 1.38 [1.31–1.45] | <0.0001 |
| Diagnostic | 0.95 [0.87–1.02] | 0.18 |
Most advanced findings ordered as no significant findings, non-advanced polyps, and advanced neoplasia.
Interpretation of odds ratio: For age, the odds ratio is 1.09, meaning that for every 5-year increase in age, there is a 9% higher odds of having an advanced finding on colonoscopy.
Recommendations for future colonoscopy
We evaluated recommendations for future colonoscopy based on findings at the current colonoscopy for each age category. Colonoscopies with follow-up recommendation “pending pathology” (N = 11,809; 32.0%) were excluded from analysis. Because recommendations were similar across the different indications (Figure S1), we combined indications together. Recommendations varied by age and findings (Figure 3). In patients without any significant findings, recommendations to continue colonoscopy decreased with age, from 97.5% (65–69 years) to 27.4% (≥85 years; p < 0.001). However, the impact of age was muted when any neoplasia, and particularly advanced neoplasia, was found. For example, among 80–84-year-olds, 39.1% without significant findings were told to continue colonoscopy versus 50.4% with non-advanced neoplasia and 75.4% with advanced neoplasia.
FIGURE 3.
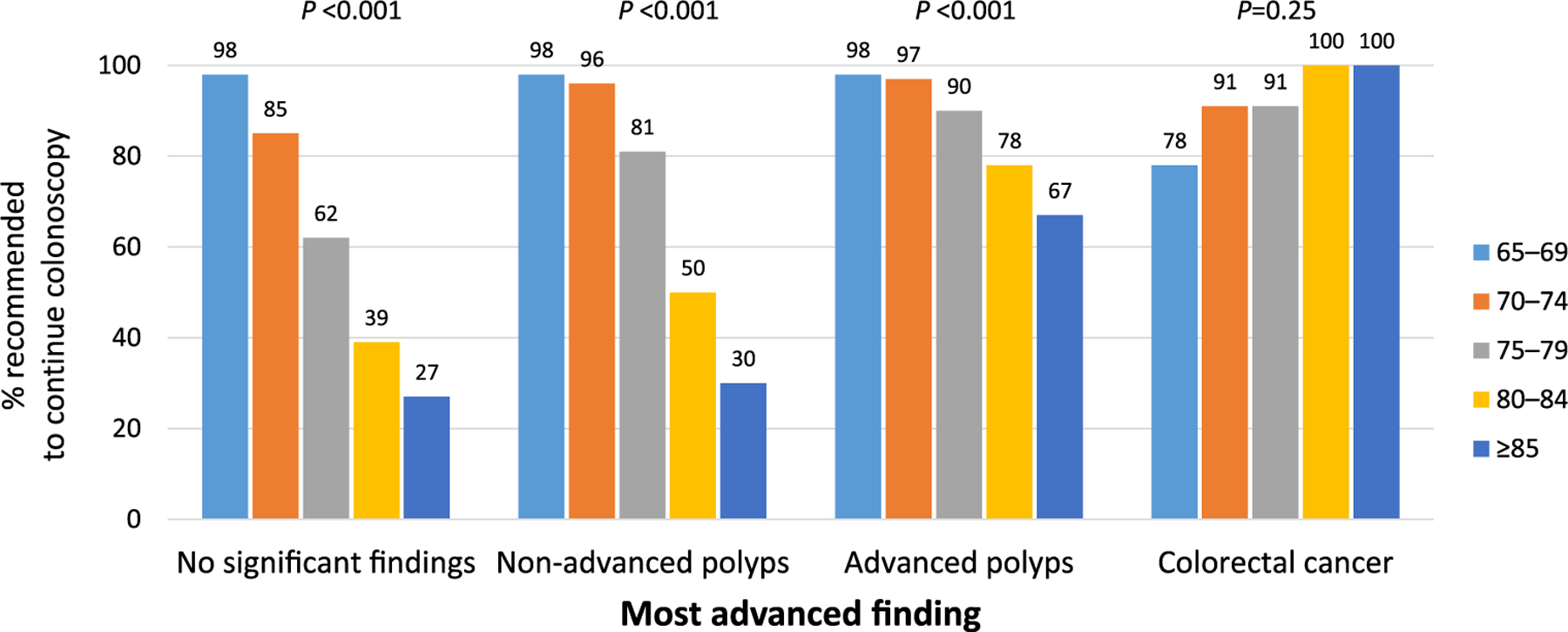
Recommendations to continue colonoscopy by age and most advanced finding on current colonoscopy. Of note, 42% of reports with no significant findings, 71% of reports with advanced polyps, and 85% of reports with colorectal cancer were excluded from this analysis due to a follow-up recommendation of “pending pathology”
DISCUSSION
In this study of a comprehensive statewide colonoscopy registry, we found that clinical findings during colonoscopy varied by indication and increased with age. Among each age group, the greatest risk of CRC occurred among diagnostic versus surveillance or screening examinations. Among screening and surveillance patients, the risk of advanced polyps increased with age; however, few older adults were found to have advanced polyps (6.2%–13.6%). Having non-advanced polyps (23.2%–42.4%) or no findings at all (45.6%–66.9%) were more common in our cohort. The vast majority of colonoscopy examinations were technically successful in terms of completeness and adequacy of bowel cleanliness, which is reassuring and in line with accepted quality metrics.19
Endoscopists were strongly influenced by the clinical findings in providing follow-up recommendations. For example, among those ≥80, 27.4%–50.4% were recommended for future colonoscopy when no significant findings or non-advanced polyps were found; this increased to 66.7%–78.4% when advanced polyps were found. Among those with non-advanced polyps, >95% of individuals aged 65 to 74 years and 80.8% of those aged 75–79 years were recommended to return for future colonoscopy. If we assume their follow-up interval would be in approximately 5–10 years as per 2012 US Multi-Society Task Force (USMSTF) post-polypectomy surveillance guidelines,20 these individuals would be anywhere from 70 to 89 years old at their next examination. Of note, this investigation did not incorporate previous polyps or CRC or stratify by family history of CRC, which could have affected the suggested future interval. In addition, a large percentage of reports provided a recommendation “pending pathology,” and this increased with the severity of the finding. Observations in our study may be an under- or overestimate of those eventually told to return for repeat colonoscopy.
This study addresses limitations of prior studies and offers some similarities and differences. A study of a colonoscopy surveillance population using the GI Quality Improvement Consortium, which gathers data from >600 US endoscopy sites, found a similar trend of decreasing frequency of recommendations to continue colonoscopy with increasing age.7 However, a greater percentage of individuals in that study were told to return for future colonoscopy at the older ages (i.e., ≥80) compared to this current NHCR study. Our study benefits from detailed NHCR data, finding a fairly high proportion of our cohort reporting a first-degree relative with CRC (23% among screening, 26.3% among surveillance) compared to the general population.21 This may be reflective of self-selection of patients with heightened awareness of CRC screening due to family history who choose to continue screening and surveillance care and participate in a colonoscopy registry.
Within the category of non-advanced polyps, there may be differences by size (i.e., <5 mm vs. 5–9 mm) in terms of risks of future advanced adenomas22; however, there is increasing evidence that the presence of one to two small polyps (≤1 cm) does not increase long-term risk of CRC or CRC death, and in fact, the risk of CRC in those with small polyps is equal or below that of the general population.23–25 Coupling the relative low risk of small polyps with competing comorbidities with advancing ages suggests that the potential harms (e.g., adverse events, psychological impact, and opportunity cost of colonoscopy taking time and distracting away from other more pressing health problems) of frequent surveillance colonoscopy in older adults with small polyps could outweigh the potential benefit. Adverse events due to colonoscopy in older adults have been well described and generally do not differ by the indication of the procedure, but by comorbidities, medications, and interventions performed (i.e., higher rates of bleeding with the removal of large polyps).4,6,26,27
Prior literature on older adults and colonoscopy has primarily focused on stopping screening and has largely ignored other indications for colonoscopy, including surveillance and diagnostic. For screening, an individualized approach that accounts for lag time to benefit from colonoscopy in older adults with the more immediate potential harms of screening has been advocated.28 However, in a survey of Veterans with normal screening results, 49% thought age should not be used in deciding when to stop and 29% were not comfortable stopping screening even in the face of little benefit.29 The most recent published US Preventive Services Task Force guidelines on CRC screening (not surveillance) recommend that the decision to screen in adults aged 76 to 85 years be individualized and that screening not be performed in those aged 86 years and older.30 In this current study, we found that 14.8% of the screening colonoscopies performed in our cohort of adults age ≥65 were between ages 75 and 84. Only 0.51% were ≥85, suggesting strong guideline-concordant care in avoiding routine screening colonoscopy in this oldest age group. Prior work using NHCR has shown that both family history of CRC and endoscopist specialty were associated with receiving a recommendation to continue screening colonoscopy.11 Understanding when and how to stop colonoscopy for indications other than screening, such as surveillance of prior polyps and for evaluation of symptoms, is important as colonoscopy is performed more commonly in older adults for these indications than screening. In terms of guidance on stopping surveillance, the 2012 USMSTF guidelines recommend that “the decision to continue surveillance should be individualized, based on assessment of benefit, risk, and comorbidities,” without listing specific ages for stopping.20 In addition to the short-term risks of the procedure, another important consideration is potential long-term harms, including cost, psychologic distress, and a long lag time to benefit of polypectomy, which may not be realized within the lifetime of some older adults.
In our study, surveillance patients tended to be older than those undergoing screening examinations; 21.1% of patients undergoing surveillance examinations were between age 75 and 84 and 1.4% were ≥ 85. In terms of decision-making around continuing versus stopping surveillance in older adults with polyps, clinicians seem to use a range of approaches.31 A focus group of primary care providers found that some deferred to specialists, others took a more active role and discussed the decision with patients and/or specialists, and others felt comfortable stopping surveillance, basing the decision on patient age, comorbidities, or life expectancy. Most found information is lacking on the benefits and harms of surveillance in older adults with prior adenomas.31 Our current study, which describes the range and distribution of findings among older adults attending surveillance colonoscopy, provides much needed data on the “benefit” in terms of detection and removal of lesions that can help inform decision-making around colonoscopy.
The strengths of our study include the use of a comprehensive, population-based registry with robust data collection, well-defined algorithms for verifying procedure indication, and pathology data linked to colonoscopy findings at the polyp level, preserving location in the colon, size, and detailed histology. Our findings that surveillance patients were more likely to be male and have a personal history of CRC, that screening patients tended to never have smoked, and diagnostic patients tended to be older and less healthy by self-report aligns with prior literature32,33 and provides additional face validity to our study. Our focus on older adults is unique, and the use of NHCR, which synergizes robust data across multiple sites, allows for analyses that would otherwise be challenging at single centers where the numbers of older adults undergoing colonoscopy would be limited.
We acknowledge certain limitations. Our sample was primarily Caucasian, reflecting the overall population of New Hampshire, in which 97.4% of people ≥65 are listed as Caucasian over a similar time period in the US census.34 Our sample was also well-educated, with the majority having attended at last some college. Follow-up recommendations provided by endoscopists within NHCR may not completely reflect conversations that occur directly with the patient outside of NHCR documentation. Endoscopists may be incorporating patient risk factors (such as prior history) that are not reflected in this data. In our cohort, there may be self-selection of those who feel strongly about colonoscopy, may have a family history of CRC, and are healthy enough to attend. Future studies that can account for comorbidities and patient life expectancy at the time of colonoscopy would be informative.35
In conclusion, this study provides real-world data on colonoscopy findings based on age and indication, suggesting that overall rates of finding advanced neoplasia are low, especially for screening examinations. The benefits of finding and removing advanced neoplasia must be considered in the context of overall life expectancy and weighed with the rare but known harms of colonoscopy, which worsen with advancing age and comorbidities. The balance of benefits and harms must also account for patient values and preferences to individualize optimal colonoscopy use in older adults.
Supplementary Material
Table S1. Characteristics of the 42,611 colonoscopy examinations performed in adults aged 65 years and older in the New Hampshire Colonoscopy Registry between 2009 and 2019.
Table S2. Most advanced finding during colonoscopy by patient age and indication of examination.
Figure S1. Recommendations to continue colonoscopy by age and indication of current colonoscopy.
Key points
Among older adults undergoing colonoscopy, there is a low incidence of advanced polyps and colorectal cancer.
Findings were lowest among screening examinations.
Why does this paper matter?
The benefits of screening colonoscopy in older adults may be small and should be weighed against the potential harms, which increase with age.
Funding information
Dr. Calderwood is supported by NCI R21 CA227776, The Dartmouth-Hitchcock Cancer Research Fellows Program, and the NCI Cancer Center Support Grant 5P30CA023108 to the Dartmouth-Hitchcock Norris Cotton Cancer Center as well as the Dartmouth Clinical and Translational Science Institute, under award number UL1TR001086 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). This project was supported in part by the Centers for Disease Control and Prevention’s National Program of Cancer Registries, cooperative agreement 5U58DP003930 awarded to the New Hampshire Department of Health and Human Services, Division of Public Health Services, Bureau of Public Health Statistics and Informatics, Office of Health Statistics and Data Management.
Footnotes
Presented in part at the Virtual Annual Meeting of the American Geriatrics Society on May 12, 2021.
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or New Hampshire Department of Health and Human Services.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
SPONSOR’S ROLE
The sponsor had no role in the design, conduct, or interpretation of this study nor in the drafting or approval of the manuscript.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.Lieberman DA, Williams JL, Holub JL, et al. Colonoscopy utilization and outcomes 2000 to 2011. Gastrointest Endosc 2014; 80:133–143. [DOI] [PubMed] [Google Scholar]
- 2.United States Census Bureau. Older people projected to out-number children for first time in U.S. history: Release Number CB18–41 Revised September 6, 2018. Accessed April 2, 2021. https://www.census.gov/newsroom/press-releases/2018/cb18-41-population-projections.html
- 3.Ko CW, Riffle S, Shapiro JA, et al. Incidence of minor complications and time lost from normal activities after screening or surveillance colonoscopy. Gastrointest Endosc 2007;65: 648–656. [DOI] [PubMed] [Google Scholar]
- 4.Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med 2009;150:849–857, W152. [DOI] [PubMed] [Google Scholar]
- 5.Ko CW, Riffle S, Michaels L, et al. Serious complications within 30 days of screening and surveillance colonoscopy are uncommon. Clin Gastroenterol Hepatol 2010;8:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day LW, Kwon A, Inadomi JM, Walter LC, Somsouk M. Adverse events in older patients undergoing colonoscopy: a systematic review and meta-analysis. Gastrointest Endosc 2011; 74:885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calderwood AH, Holub JL, Greenwald DA, Robertson DJ. Yield and practice patterns of surveillance colonoscopy among older adults: an analysis of the GI Quality Improvement Consortium. Am J Gastroenterol 2019;114:1811–1819. [DOI] [PubMed] [Google Scholar]
- 8.QuickStats: Colorectal Cancer Screening Among Adults Aged 50–75 Years, by Race/Ethnicity — National Health Interview Survey, United States, 2000–2015. MMWR Morb Mortal Wkly Rep 2016;65:1042. 10.15585/mmwr.mm6538a6. [DOI] [PubMed] [Google Scholar]
- 9.Greene MA, Butterly LF, Goodrich M, et al. Matching colonoscopy and pathology data in population-based registries: development of a novel algorithm and the initial experience of the New Hampshire Colonoscopy Registry. Gastrointest Endosc 2011;74:334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butterly LF, Siegel RL, Fedewa S, Robinson CM, Jemal A, Anderson JC. Colonoscopy outcomes in average-risk screening equivalent young adults: data from the New Hampshire Colonoscopy Registry. Am J Gastroenterol 2021.116:171–179. [DOI] [PubMed] [Google Scholar]
- 11.Calderwood AH, Anderson JC, Robinson CM, Butterly LF. Endoscopist specialty predicts the likelihood of recommending cessation of colorectal cancer screening in older adults. Am J Gastroenterol 2018;113:1862–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson JC, Calderwood AH, Christensen BC, Robinson CM, Amos CI, Butterly L. Smoking and other risk factors in individuals with synchronous conventional high-risk adenomas and clinically significant serrated polyps. Am J Gastroenterol 2018; 113:1828–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson JC, Butterly LF, Goodrich M, Robinson CM, Weiss JE. Differences in detection rates of adenomas and serrated polyps in screening versus surveillance colonoscopies, based on the New Hampshire Colonoscopy Registry. Clin Gastroenterol Hepatol 2013;11:1308–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson JC, Robinson CM, Butterly LF. Increased risk of metachronous large serrated polyps in individuals with 5- to 9-mm proximal hyperplastic polyps: data from the New Hampshire Colonoscopy Registry. Gastrointest Endosc 2020;92:387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson JC, Butterly LF, Robinson CM, Goodrich M, Weiss JE. Impact of fair bowel preparation quality on adenoma and serrated polyp detection: data from the New Hampshire colonoscopy registry by using a standardized preparation-quality rating. Gastrointest Endosc 2014;80:463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butterly LF, Nadel MR, Anderson JC, et al. Impact of colonoscopy bowel preparation quality on follow-up interval recommendations for average-risk patients with Normal screening colonoscopies: data from the New Hampshire Colonoscopy Registry. J Clin Gastroenterol 2020;54:356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrew AS, Parker S, Anderson JC, et al. Risk factors for diagnosis of colorectal cancer at a late stage: a population-based study. J Gen Intern Med 2018;33:2100–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis Spring-Verlag; 2001. [Google Scholar]
- 19.Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015;81:31–53. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844–857. [DOI] [PubMed] [Google Scholar]
- 21.Ramsey SD, Yoon P, Moonesinghe R, Khoury MJ. Population-based study of the prevalence of family history of cancer: implications for cancer screening and prevention. Genet Med 2006; 8:571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson JC, Rex DK, Robinson C, Butterly LF. Association of small versus diminutive adenomas and the risk for metachronous advanced adenomas: data from the New Hampshire Colonoscopy Registry. Gastrointest Endosc 2019;90:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loberg M, Kalager M, Holme O, Hoff G, Adami HO, Bretthauer M. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med 2014;371:799–807. [DOI] [PubMed] [Google Scholar]
- 24.Click B, Pinsky PF, Hickey T, Doroudi M, Schoen RE. Association of colonoscopy adenoma findings with long-term colorectal cancer incidence. JAMA 2018;319:2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JK, Jensen CD, Levin TR, et al. Long-term risk of colorectal cancer and related death after adenoma removal in a large, community-based population. Gastroenterology 2020;158:884–94.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kothari ST, Huang RJ, Shaukat A, et al. ASGE review of adverse events in colonoscopy. Gastrointest Endosc 2019;90: 863–876. [DOI] [PubMed] [Google Scholar]
- 27.García-Albéniz X, Hsu J, Bretthauer M, Hernán MA. Effectiveness of screening colonoscopy to prevent colorectal cancer among Medicare beneficiaries aged 70 to 79 years: a prospective observational study. Ann Intern Med 2017;166:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee KT, Harris RP, Schoenborn NL. Individualized approach to cancer screening in older adults. Clin Geriatr Med 2018;34: 11–23. [DOI] [PubMed] [Google Scholar]
- 29.Maratt JK, Calderwood AH. Colorectal cancer screening and surveillance colonoscopy in older adults. Curr Treat Options Gastroenterol 2019;17:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Force USPST, Bibbins-Domingo K, Grossman DC, et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016;315:2564–2575. [DOI] [PubMed] [Google Scholar]
- 31.Schoenborn NL, Boyd CM, Massare J, Park R, Choi Y, Pollack CE. Primary care clinician decision-making around surveillance colonoscopies in older adults with prior adenomas. J Am Board Fam Med 2020;33:796–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Debarros M, Steele SR. Colorectal cancer screening in an equal access healthcare system. J Cancer 2013;4:270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss JM, Smith MA, Pickhardt PJ, et al. Predictors of colorectal cancer screening variation among primary-care providers and clinics. Am J Gastroenterol 2013;108:1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Census Bureau. ACS 5-Year Estimates Subject Tables) Table S0103 Population 65 Years and Over in the United States 2019. Accessed August 23, 2021. https://data.census.gov/cedsci/table?q=age&g=0400000US33&tid=ACSST5Y2019.S0103
- 35.Schonberg MA, Li V, Marcantonio ER, Davis RB, McCarthy EP. Predicting mortality up to 14 years among community-dwelling adults aged 65 and older. J Am Geriatr Soc 2017;65:1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of the 42,611 colonoscopy examinations performed in adults aged 65 years and older in the New Hampshire Colonoscopy Registry between 2009 and 2019.
Table S2. Most advanced finding during colonoscopy by patient age and indication of examination.
Figure S1. Recommendations to continue colonoscopy by age and indication of current colonoscopy.


