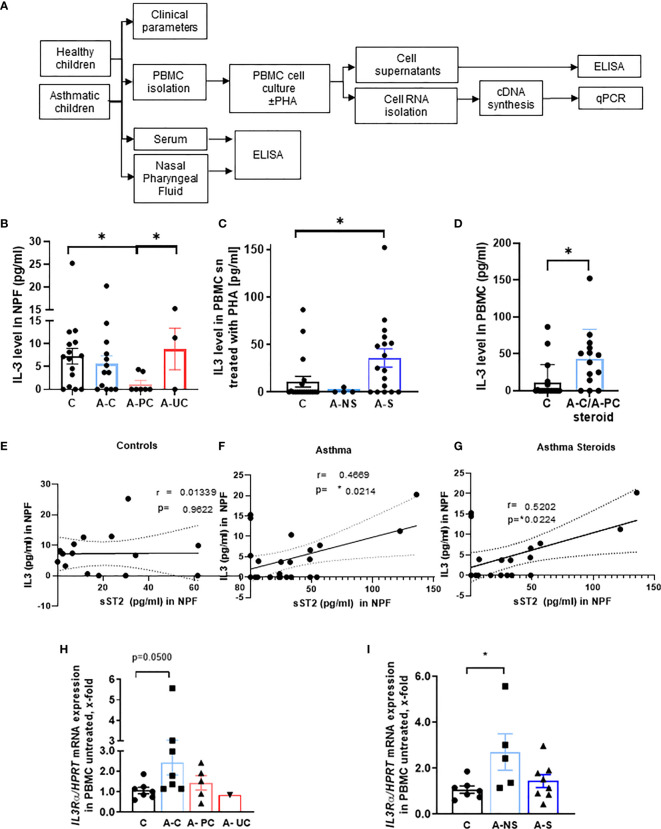
Figure 1.
Role of IL-3 in asthmatic patients. (A) Experimental design of the study PreDicta on the children cohorts analyzed in this study. (B) ELISA analysis of IL-3 levels in the nasopharyngeal fluid (NPF) from control preschooler and asthmatic preschooler subgrouped into asthma controlled (A–C), asthma partially controlled (A-PC), and asthma uncontrolled (A-UC) (GINA 2009) (n = 15, 13, 7, 3). (C) ELISA analysis of IL-3 levels in the supernatant from peripheral blood mononuclear cells (PBMCs) isolated from healthy control and asthmatic children and cultured with phytohemagglutinin (PHA) for 24 h. Asthmatic children were subgrouped in accordance to the medications used: non-steroids (NS) and steroids (S) (n = 19, 4, 18; p = 0.028). (D) Analysis (shown in panel C) considering steroid-treated asthmatic children controlled and partially controlled (n = 19, 14). (E–G) IL-3 in NPF from control (E) (n = 15) and asthmatic preschoolers (F) (n = 24) were correlated with the correspondent sST2 in NPF. A significant positive correlation was maintained when only the asthmatic children treated with steroids were correlated (G) (n = 19) (Pearson’s correlation test). (H) IL3Ra/HPRT mRNA expression in PBMCs of controls and asthmatic children subgrouped into asthma controlled (A–C), asthma partially controlled (A-PC), and asthma uncontrolled (A-UC) (n = 7, 7, 5, 1) and (I) in asthmatic children treated with (A-S) or without steroids (A-NS) at the baseline visit (n = 7, 5, 8). Data are presented as means ± SEMs. Two-tailed Student’s t-test or Kruskal–Wallis test was used to calculate statistical significance. *p ≤ 0.05.