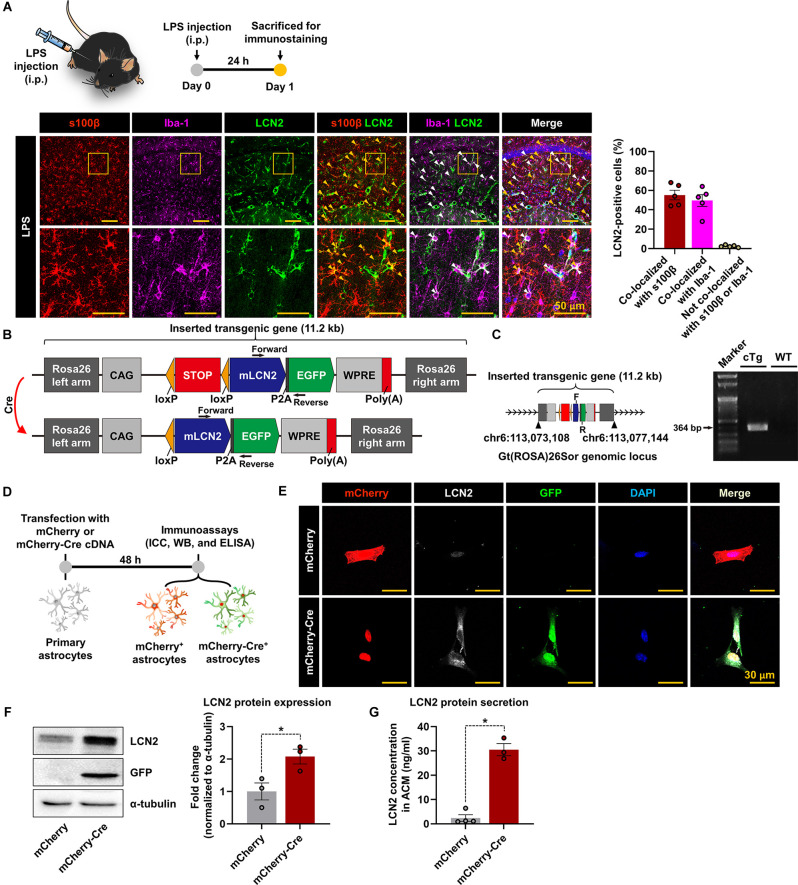
Figure 1.
The generation of LCN2-cTg mice. (A) A schematic showing the experimental timelines. LPS-injected brain sections containing the hippocampal region were subjected to immunofluorescence analysis to assess the expression of LCN2 (green) in s100β-positive (red, an astrocytes marker) or Iba-1-positive (magenta, a microglial marker) cells. LCN2 expression was co-localized with either astrocytes (yellow arrowheads), microglia (white arrowheads), and nuclei (blue). The quantification graph is presented as means ± SEM (n = 5, right). (B) Schematic design of LCN2-cTg mice. Inserted transgenic genes were designed to express mouse LCN2 (mLCN2) and EGFP with the CAG promoter only in Cre-expressing cells. (C) Whole-genome sequencing (WGS) results, indicating the location of the inserted transgenic gene in the Rosa26 locus (left); F, forward; R, reverse. Genotype PCR results with designed primer pair (right). (D) Diagram showing the timeline of experimentation. Cultured astrocytes were transfected with a mCherry or mCherry-Cre expression construct. After 48 h, immunocytochemistry (ICC) (E), Western blot (WB) (F), and ELISA (G) of transfected cells or culture media were conducted. (E) Microscopic data showing the colocalization of mCherry, LCN2, and GFP in mCherry-Cre-transfected astrocytes. (F) Protein levels of LCN2 and GFP, assessed by Western blotting in transfected astrocytes (n = 3). (G) The extracellular LCN2 protein concentration was measured from the astrocyte-conditioned medium (ACM, n = 3–4). Data are presented as the means ± SEM (*p < 0.05, between the indicated groups).