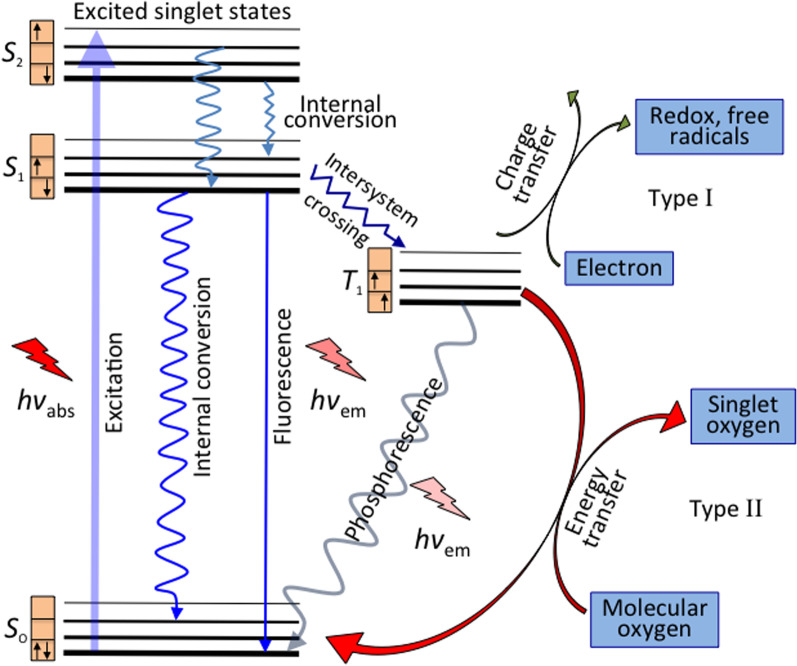
Fig. 3.
Generation of reactive oxygen species and excited states of the photosensitizer. Light promotes the excitation of an electron from a low-energy singlet state (So) to high-energy singlet states (S1,2). Such states can lose their energy via fluorescence (radiative emission, light) or internal conversion (non-radiative emission, heat). The spin flipping of the high-energy electron takes places via intersystem crossing, which leads to a long-lived excited triplet state (T1). Type I and II reactions favor the formation of free radicals and singlet oxygen (1O2), respectively, in the presence of 3O2. Significantly modified from Ref. [11]