TABLE 1.
Reaction condition optimization studies.
a
| Entry | Base | Solvent | Yield b (%) |
|---|---|---|---|
| 1 | Cs2CO3 | EA | 15 |
| 2 | Et3N | EA | — |
| 3 | NaHCO3 | EA | 38 |
| 4 | Na2CO3 | EA | 66 |
| 5 | NaOH | EA | 11 |
| 6 | K2CO3 | EA | 80 |
| 7 | NH4HCO3 | EA | 82 |
| 8 | NH4HCO3 | DCM | 65 |
| 9 | NH4HCO3 | CHCl3 | 52 |
| 10 | NH4HCO3 | Et2O | 61 |
| 11 | NH4HCO3 | Toluene | 73 |
| 12 | NH4HCO3 | DCE | 70 |
| 13 | NH4HCO3 | MTBE | 85 |
| 14 | NH4HCO3 | CCl4 | 54 |
| 15 c | NH4HCO3 | MTBE | 88 |
| 16 d | NH4HCO3 | MTBE | 80 |
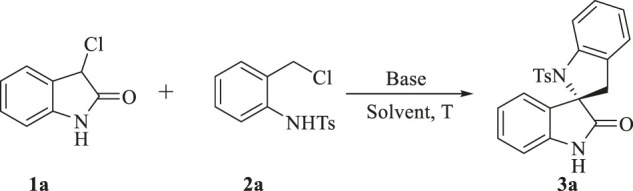
All reactions were conducted with 0.4 mmol of 1a (1.0 equiv.), 0.44 mmol of 2a (1.1 equiv.), and 1.2 mmol of base in 4.0 ml of solvent at rt.
Yield of isolated compound 3a after chromatography.
The reaction was conducted at 40°C.
The reaction was conducted at 50°C.
All the reactions were conducted with 0.4 mmol of 1 (1.0 equiv.), 0.44 mmol of 2 (1.1 equiv.) and 1.2 mmol of base in MTBE (4.0 mL) at 40°C. Yields are those of the isolated products 3a–3n after column chromatography.
