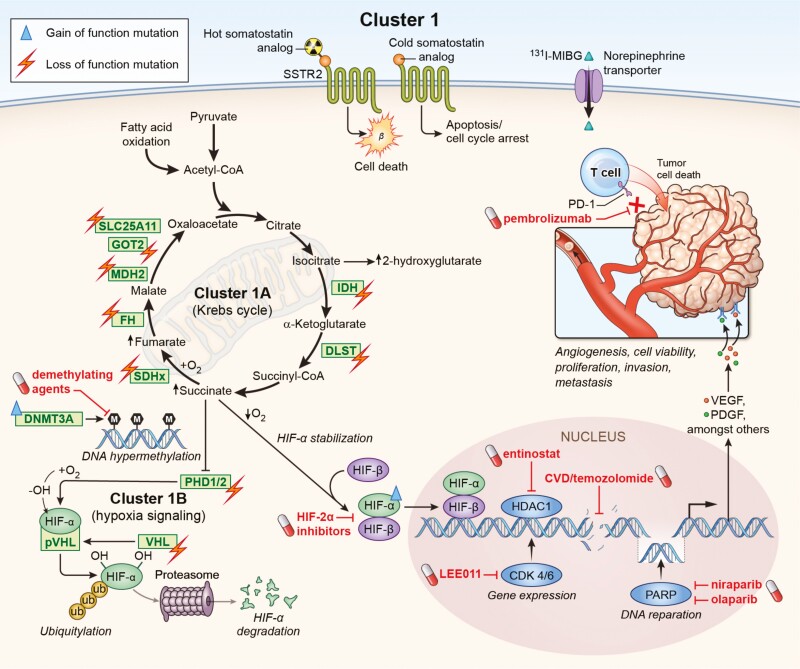
Figure 1.
Gene mutations impairing either Krebs cycle (cluster 1A) or hypoxia-signaling (cluster 1B) are associated with the development of pseudohypoxic cluster 1 PPGLs. These molecular changes offer potential targets for personalized medicine. Loss of function mutations in SDHA[AF2]/B/C/D, FH, MDH2, IDH, GOT2, SLC25A11, and DLST affect the Krebs cycle, resulting in severe impairment of mitochondrial oxidative phosphorylation and an accumulation of oncometabolites such as succinate. Accumulation of these oncometabolites as well as mutations (PDH1/2, VHL) leading to a decreased degradation of HIF-α result in an enhanced expression and stabilization of HIF-α. Moreover, gain-of-function mutation in HIF2A underlines the importance of hypoxia signaling in cluster 1 PPGLs. Highlighted in red are potential drugs that could be used to negate the molecular changes in cluster 1 PPGLs, which are in preclinical and clinical evaluation. In addition, targeting the somatostatin receptor (possibly higher expression compared to cluster 2) or the norepinephrine transporter (possibly lower expression compared to cluster 2) can be used to treat these tumors. Further approaches address immune checkpoints such as PD-1 or DNA repair mechanisms.