TABLE 2.
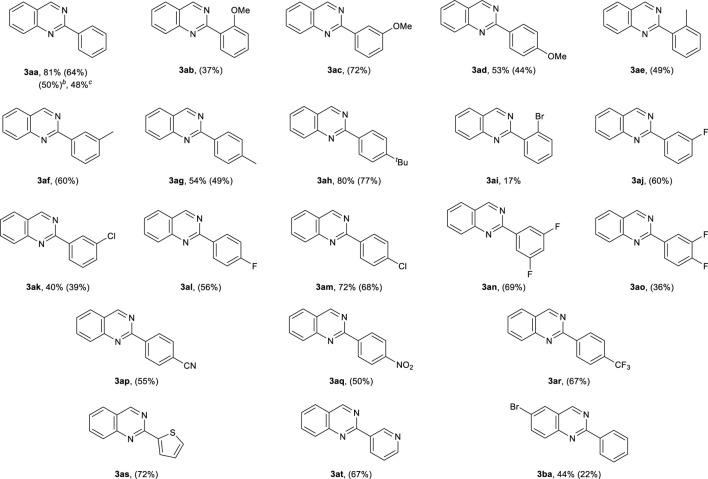
Reaction scope for the metal-free/oxidative synthesis of 2-substituted quinazolines.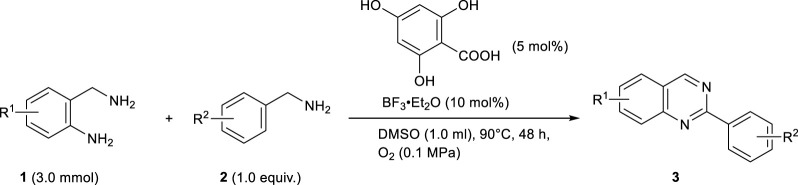
|
Yields were determined by 1H NMR spectroscopy (isolated yields).
Reaction conditions: 1a (10.0 mmol), 2a (1.0 equiv.), 4,6-dihydroxysalicylic acid (5 mol%), BF3‧Et2O (10 mol%), DMSO (2.5 ml), 90°C, 48 h, O2 (0.1 MPa). c Reaction conditions: 1a (3.0 mmol), 2a (3.0 mmol), salicylic acid (10 mol%), 4A MS (100 mg), DMSO (1.0 ml), 90°C, 5 days, O2 (0.1 MPa).
