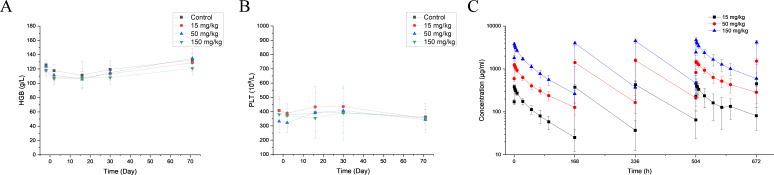
Figure 6.
BR105 can be safely administered intravenously in non-human primates. BR105 was administered intravenously to cynomolgus monkeys at 15, 50, and 150 mg/kg (five males and five females for each group) one time per week for 4 weeks. Following the dosing period, two animals/sex per group were maintained for a 6-week recovery period. (A, B) Peripheral blood was collected for hematology during the pre-dose phase; on Days 2 and 16 of the dosing phase; and on Days 30 and 71 of the recovery period. (C) Serum concentration-time profiles of BR105 following intravenous repeat doses in cynomolgus monkeys (semi-logatithmic). Values are presented as mean ± SD.