Abstract
Dogs and cats are important hosts and reservoirs of many viral, bacterial, protozoal, and helminthic pathogens transmitted by arthropods, including some of zoonotic concern. By sharing the same environment, these companion animals play an important role in the transmission of zoonotic pathogens to humans in various regions and socioeconomic contexts. While canine and feline vector-borne diseases (VBD) are of major concern in wealthy regions (e.g. Europe and North America), less attention has been received in developing countries such as those in Southeast Asia (SEA). This review provides summarized and updated information on canine and feline VBD with emphasis on those of zoonotic concern in SEA. Of these, zoonotic bacteria (i.e. Bartonella henselae, Bartonella clarridgeiae, and Rickettsia felis) and filarial nematodes (i.e. Brugia malayi, Dirofilaria repens, and Dirofilaria immitis) stand out as the most important in veterinary and human medicine. Additionally, the recent finding of Leishmania infantum in dogs in SEA raised more concerns about the spreading of this zoonotic agent in this region. Further epidemiological surveys, especially in countries with extremely scant information such as Cambodia, Laos, Myanmar, and Timor-Leste are advocated. Additionally, effective control measures of canine and feline VBD as well as their arthropod vectors should be simultaneously performed for the management of zoonotic infections.
Keywords: Cats, Dogs, Southeast Asia, Vector-borne diseases, Zoonotic
Abbreviations: GPELP, Global Programme to Eliminate Lymphatic Filariasis; LF, lymphatic filariasis; MDA, mass drug administration; POC, point-of-care; s.l., sensu lato; SEA, Southeast Asia; VBD, vector-borne diseases; VBP, vector-borne pathogens
Graphical abstract
Highlights
-
•
Many canine and feline vector-borne infections affect animals and humans in Southeast Asia.
-
•
Ticks, fleas and mosquitoes are the most common vectors transmitting pathogens to dogs, cats and humans in Southeast Asia.
-
•
Bartonella henselae, Rickettsia felis and Dirofilaria repens are of concern to human health in this region.
-
•
Collaboration between governments and researchers is encouraged for a better management of vector-borne diseases.
1. Introduction
Southeast Asia (SEA) comprises 11 countries (Brunei Darussalam, Cambodia, Indonesia, Laos, Malaysia, Myanmar, the Philippines, Singapore, Thailand, Timor-Leste, and Vietnam) (Fig. 1), and is home to more than 650 million people with approximately 49% of the population living in rural areas (by 2018) (The World Bank, 2019). In recent decades, SEA countries have been experiencing the fastest-ever economic transformation, which also leads to living standard and health improvements (Fukugawa, 2018). However, this region is still marked by significant social disparities, and remains as a hotspot of many infectious diseases such as dengue fever, malaria, and rabies, which are life-threatening to millions of people (Shepard et al., 2013; Hotez et al., 2015). For example, in spite of the decreasing trend, it is estimated that more than 1,453,000 malaria cases occurred in SEA in 2018, with 2,298 estimated deaths (World Health Organization, 2019).
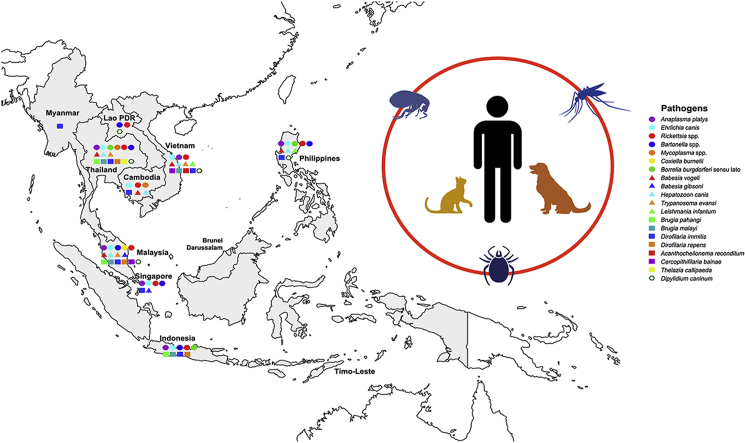
Fig. 1.
The distribution of vector-borne pathogens in dogs and cats from Southeast Asia.
In addition to the above-mentioned diseases, many zoonotic vector-borne diseases (VBD) have been reported in SEA (Colella et al., 2020; Irwin & Jefferies, 2004; Low et al., 2020), and for some of these dogs and cats play a significant reservoir role. For instance, zoonotic vector-borne pathogens (VBP) such as Rickettsia felis and Bartonella henselae have been reported in patients having previous contact with dogs, cats and/or ticks and fleas (Edouard et al., 2014; Noopetch et al., 2018). Dirofilaria immitis, the causative agent of canine and feline heartworm disease and human pulmonary dirofilariasis (Dantas-Torres & Otranto, 2020), is widely distributed in SEA being reported in dogs from Malaysia, Singapore, and southern Thailand (Colella et al., 2020; Kamyingkird et al., 2017; Lau et al., 2017). Furthermore, although SEA is considered as outside the geographical distribution area of Leishmania infantum, the presence of this zoonotic pathogen has been reported in dogs in this region (Colella et al., 2020).
The awareness regarding the importance of canine and feline VBD is continuously increasing in wealthy regions (e.g. Europe and North America), but less attention has been received in developing regions like SEA. In such regions, there is limited or no access of dogs and cats living in poor suburban or rural areas (Fig. 2A) to veterinary services and preventive measures, thus increasing their risk of acquiring VBP (Dantas-Torres et al., 2020; Otranto et al., 2017). Additionally, the limited availability of financial resources and laboratory facilities in some countries in SEA have historically impaired the scientific knowledge about canine and feline VBD in this region, which certainly has a negative impact on the establishment of appropriate strategies to mitigate zoonotic VBD. Nonetheless, recent multicenter collaborative research has positively impacted on our knowledge on several VBD in SEA, filling some research gaps and uncovering others (Colella et al., 2020). In this perspective, the present article is aimed to provide an update review on VBP and their vectors affecting dogs and cats in SEA, with the main focus on those of zoonotic concern.
Fig. 2.
Free-roaming dogs in a rural area of Vietnam (A). Heavy infestation of Rhipicephalus sanguineus (sensu lato) in a pet dog (B). A female of Ctenocephalides orientis with spermatheca (white arrowhead), strongly rounded anterior margin of the head (arrowhead), 2 setae on the lateral metonotal area (white arrows), and 7 setae-bearing notches on the dorsal margin of the hind tibia (arrows) (C).
2. Arthropod vectors of VBP affecting dogs and cats in SEA: what do we know
Ticks, fleas, and mosquitoes represent the most common arthropod vectors transmitting pathogens to dogs and cats in SEA (Irwin & Jefferies, 2004; Colella et al., 2020). Numerous VBP have been detected in dogs and cats as well as in their associated arthropods in this region (Table 1). The brown dog ticks, Rhipicephalus sanguineus (sensu lato) (Fig. 2B) are the most prevalent ticks found on dogs and, to a lesser extent, cats in SEA (Petney et al., 2019). These ticks act as vectors of many pathogens (e.g. Anaplasma platys, Ehrlichia canis, Babesia vogeli, and Hepatozoon canis) affecting dogs, cats, and humans in tropical and subtropical regions (Dantas-Torres & Otranto, 2015). There have been many investigations indicating the presence of two divergent lineages of this tick species (i.e. the tropical and the temperate lineage) in different parts of the world (Dantas-Torres et al., 2013; Nava et al., 2018), and the pathogens transmitted by ticks of these two lineages may also differ (Moraes-Filho et al., 2015). A recent study showed that R. sanguineus (s.l.) ticks circulating in SEA belong to the tropical lineage (Nguyen et al., 2020b). Additionally, other tick species infesting dogs such as Rhipicephalus haemaphysaloides, Haemaphysalis hystricis, Haemaphysalis wellingtoni, and Haemaphysalis papuana have been reported also sporadically (Durden et al., 2008; Kolonin, 1995; Tanskul et al., 1983). However, the role of these ticks as vectors of pathogens in the transmission to dogs in SEA remains unknown.
Table 1.
Vector-borne pathogens detected in dogs, cats and/or arthropod vectors in Southeast Asia and their zoonotic relevance (i.e. nil, low, moderate, high).
| Pathogen | Zoonotic relevance | Isolation source | Country | Reference |
|---|---|---|---|---|
| Bacteria | ||||
| Anaplasma platys | Low | Dog | Indonesia | Faizal et al. (2019) |
| Malaysia | Mokhtar et al. (2013) | |||
| Philippines | Ybañez et al. (2016) | |||
| Singapore | Colella et al. (2020) | |||
| Thailand | Pinyoowong et al. (2008) | |||
| Vietnam | Chien et al. (2019) | |||
| Cat | Thailand | Salakij et al. (2012) | ||
| Ctenocephalides felis (from dog) | Laos | Calvani et al. (2020) | ||
| Rhipicephalus sanguineus (s.l.) (from dogs) | Laos | Nguyen et al. (2020a) | ||
| Malaysia | Low et al. (2018) | |||
| Philippines | Ybañez et al. (2012) | |||
| Thailand | Foongladda et al. (2011) | |||
| Ehrlichia canis | Low | Dog | Cambodia | Inpankaew et al. (2016) |
| Indonesia | Colella et al. (2020) | |||
| Malaysia | Nazari et al. (2013) | |||
| Philippines | Corales et al. (2014) | |||
| Singapore | Colella et al. (2020) | |||
| Thailand | Huggins et al. (2019) | |||
| Vietnam | Colella et al. (2020) | |||
| R. sanguineus (s.l.) (from dogs) | Malaysia | Low et al. (2018) | ||
| Philippines | Ybañez et al. (2012) | |||
| Thailand | Foongladda et al. (2011) | |||
| Vietnam | Nguyen et al. (2019) | |||
| Rickettsia felis | High | Dog | Cambodia | Inpankaew et al. (2016) |
| C. felis (from dogs) | Indonesia | Nguyen et al. (2020b) | ||
| Laos | Kernif et al. (2012) | |||
| Malaysia | Kernif et al. (2012) | |||
| Philippines | Wolf and Reeves (2012) | |||
| Thailand | Nguyen et al. (2020b) | |||
| Vietnam | Nguyen et al. (2020b) | |||
| C. felis (from cats) | Laos | Varagnol et al. (2009) | ||
| Indonesia | Nguyen et al. (2020b) | |||
| Philippines | Nguyen et al. (2020b) | |||
| Vietnam | Nguyen et al. (2020b) | |||
| Ctenocephalides orientis (from dogs) | Indonesia | Nguyen et al. (2020b) | ||
| Laos | Kernif et al. (2012) | |||
| Malaysia | Kernif et al. (2012) | |||
| Pulex irritans (from dog) | Laos | Calvani et al. (2020) | ||
| R. sanguineus (s.l.) (from dogs) | Philippines | Nguyen et al. (2020b) | ||
| Heterodoxus spiniger (from dogs) | Laos | Nguyen et al. (2020a) | ||
| Rickettsia asembonensis | Nil | C. felis (from dogs) | Laos | Nguyen et al. (2020a) |
| Philippines | Nguyen et al. (2020b) | |||
| C. orientis (from dogs) | Indonesia | Nguyen et al. (2020b) | ||
| Malaysia | Low et al. (2017) | |||
| Philippines | Nguyen et al. (2020b) | |||
| Thailand | Nguyen et al. (2020b) | |||
| Vietnam | Nguyen et al. (2020b) | |||
| R. sanguineus (s.l.) (from dogs) | Indonesia | Nguyen et al. (2020b) | ||
| Malaysia | Low et al. (2017) | |||
| “Candidatus Rickettsia senegalensis” | Nil | C. orientis (from dogs) | Thailand | Nguyen et al. (2020b) |
| Rickettsia sp. genotype RF2125 | Nil | Cat | Thailand | Phoosangwalthong et al. (2018) |
| C. orientis (from dogs) | Laos | Calvani et al. (2020) | ||
| Ctenocephalides canis? (from dogs) | Thailand | Parola et al. (2003b) | ||
| Bartonella henselae | High | Dog | Philippines | Singer et al. (2020) |
| Cat | Indonesia | Marston et al. (1999) | ||
| Malaysia | Hassan et al. (2017) | |||
| Philippines | Chomel et al. (1999) | |||
| Singapore | Nasirudeen and Thong (1999) | |||
| Thailand | Maruyama et al. (2001) | |||
| C. felis (from dogs/cats) | Malaysia | Mokhtar and Tay (2011) | ||
| C. felis (from cats) | Thailand | Parola et al. (2003b) | ||
| C. canis? (from cats) | Thailand | Foongladda et al. (2011) | ||
| Bartonella clarridgeiae | High | Dog | Thailand | Billeter et al. (2012) |
| Cat | Indonesia | Marston et al. (1999) | ||
| Philippines | Chomel et al. (1999) | |||
| Thailand | Maruyama et al. (2001) | |||
| C. felis (from dogs) | Laos | Varagnol et al. (2009) | ||
| Thailand | Billeter et al. (2012) | |||
| C. felis (from dogs/cats) | Malaysia | Mokhtar and Tay (2011) | ||
| C. felis (from cats) | Laos | Calvani et al. (2020) | ||
| Thailand | Parola et al. (2003b) | |||
| C. orientis (from dogs) | Laos | Kernif et al. (2012) | ||
| C. canis? (from dog) | Thailand | Billeter et al. (2012) | ||
| Bartonella koehlerae | High | Cat | Thailand | Assarasakorn et al. (2012) |
| C. felis (from cats) | Thailand | Assarasakorn et al. (2012) | ||
| Bartonella elizabethae | High | Dog | Thailand | Bai et al. (2010) |
| Bartonella quintana | High | Dog | Thailand | Bai et al. (2010) |
| Bartonella rochalimae | High | P. irritans (from dogs) | Laos | Calvani et al. (2020) |
| R. sanguineus (s.l.) (from dogs) | Thailand | Billeter et al. (2012) | ||
| Bartonella vinsonii subsp. arupensis | High | Dog | Thailand | Bai et al. (2010) |
| Bartonella vinsonii subsp. berkhoffii | High | Dog | Thailand | Suksawat et al. (2001) |
| Cat | Thailand | Srisanyong et al. (2016) | ||
| Bartonella vinsonii subsp. vinsonii | Nil | C. felis (from dogs) | Thailand | Billeter et al. (2012) |
| Mycoplasma haemofelis | Nil | Cat | Thailand | Assarasakorn et al. (2012) |
| C. felis (from cats) | Thailand | Assarasakorn et al. (2012) | ||
| “Candidatus Mycoplasma haemominutum” | Nil | Dog | Thailand | Liu et al. (2016) |
| Cat | Thailand | Assarasakorn et al. (2012) | ||
| C. felis (from cats) | Thailand | Assarasakorn et al. (2012) | ||
| “Candidatus Mycoplasma turicensis” | Nil | Dog | Thailand | Huggins et al. (2019) |
| Cat | Thailand | Do et al. (2020) | ||
| “Candidatus Mycoplasma haematoparvum” | Nil | Dog | Cambodia | Inpankaew et al. (2016) |
| Thailand | Kaewmongkol et al. (2017) | |||
| Mycoplasma haemocanis | Nil | Dog | Cambodia | Inpankaew et al. (2016) |
| Thailand | Kaewmongkol et al. (2017) | |||
| Coxiella burnetii | High | Dog | Malaysia | Tukur et al. (2019) |
| R. sanguineus (s.l.) (from dogs) | Malaysia | Watanabe et al. (2015) | ||
| Borrelia burgdorferi (s.l.) | High | Dog | Indonesia | Colella et al. (2020) |
| Philippines | Colella et al. (2020) | |||
| Thailand | Sthitmatee et al. (2016) | |||
| Protozoans | ||||
| Babesia vogeli | Nil | Dog | Cambodia | Inpankaew et al. (2016) |
| Malaysia | Prakash et al. (2018b) | |||
| Philippines | Galay et al. (2018) | |||
| Thailand | Piratae et al. (2015) | |||
| Cat | Thailand | Simking et al. (2010) | ||
| R. sanguineus (s.l.) (from dogs) | Malaysia | Prakash et al. (2018b) | ||
| Philippines | Galay et al. (2018) | |||
| Vietnam | Nguyen et al. (2019) | |||
| Babesia gibsoni | Nil | Dog | Malaysia | Mokhtar et al. (2013) |
| Singapore | Colella et al. (2020) | |||
| R. sanguineus (s.l.) (from dogs) | Malaysia | Prakash et al. (2018b) | ||
| Hepatozoon canis | Nil | Dog | Cambodia | Inpankaew et al. (2016) |
| Malaysia | Prakash et al. (2018a) | |||
| Philippines | Galay et al. (2018) | |||
| Thailand | Piratae et al. (2015) | |||
| Vietnam | Colella et al. (2020) | |||
| Cat | Thailand | Jittapalapong et al. (2006) | ||
| R. sanguineus (s.l.) (from dogs) | Malaysia | Prakash et al. (2018b) | ||
| Philippines | Galay et al. (2018) | |||
| Thailand | Nguyen et al. (2020b) | |||
| Vietnam | Nguyen et al. (2019) | |||
| R. sanguineus (s.l.) (from cats) | Philippines | Nguyen et al. (2020b) | ||
| Trypanosoma evansi | Low | Dog | Malaysia | Rajamanickam et al. (1985) |
| Thailand | Barameechaithanun et al. (2009) | |||
| Vietnam | Bui et al. (2020) | |||
| Leishmania infantum | High | Dog | Philippines | Colella et al. (2020) |
| Vietnam | Colella et al. (2020) | |||
| Helminths | ||||
| Brugia pahangi | Low | Dog | Malaysia | Colella et al. (2020) |
| Thailand | Satjawongvanit et al. (2019) | |||
| Cat | Indonesia | Palmieri et al. (1985) | ||
| Malaysia | Tan et al. (2011) | |||
| Thailand | Nuchprayoon et al. (2006) | |||
| Brugia malayi | High | Dog | Thailand | Satjawongvanit et al. (2019) |
| Vietnam | Colella et al. (2020) | |||
| Cat | Indonesia | Palmieri et al. (1985) | ||
| Malaysia | Al-Abd et al. (2015) | |||
| Thailand | Chansiri et al. (2002) | |||
| Dirofilaria immitis | High | Dog | Cambodia | Inpankaew et al. (2016) |
| Indonesia | Erawan et al. (2018) | |||
| Malaysia | Lau et al. (2017) | |||
| Myanmar | Aung (2014) | |||
| Philippines | Theis et al. (2008) | |||
| Singapore | Colella et al. (2020) | |||
| Thailand | Kamyingkird et al. (2017) | |||
| Vietnam | Colella et al. (2020) | |||
| Cat | Indonesia | Colella et al. (2020) | ||
| Malaysia | Mak et al. (1980) | |||
| Thailand | Kamyingkird et al. (2017) | |||
| Dirofilaria repens | High | Cat | Indonesia | Palmieri et al. (1985) |
| Malaysia | Al-Abd et al. (2015) | |||
| Thailand | Wongkamchai et al. (2014) | |||
| Thelazia callipaeda | Moderate | Dog | Thailand | Bhaibulaya et al. (1970) |
| Acanthocheilonema reconditum | Nil | Cat | Thailand | Wongkamchai et al. (2014) |
| Cercopithifilaria bainae | Nil | C. felis (from cats) | Vietnam | V.-L. Nguyen (unpublished data) |
| R. sanguineus (s.l.) (from dogs) | Malaysia | Latrofa et al. (2014) | ||
| Dipylidium caninum | Low | Dog | Malaysia | Ngui et al. (2014) |
| Philippines | Colella et al. (2020) | |||
| Thailand | Rojekittikhun et al. (2014) | |||
| Vietnam | Nguyen et al. (2015) | |||
| Cat | Laos | Scholz et al. (2003) | ||
| Malaysia | Mohd Zain et al. (2013) | |||
| Thailand | Jittapalapong et al. (2007) | |||
| C. felis (from cats) | Malaysia | Low et al. (2017) | ||
| Felicola subrostratus (from cats) | Malaysia | Low et al. (2017) | ||
A “?” indicates uncertain data on species identification of C. canis.
The cosmopolitan cat flea Ctenocephalides felis is commonly found on dogs and cats around the world, including SEA (Lawrence et al., 2019). This flea species is involved in the transmission of many zoonotic bacterial pathogens (e.g. B. henselae, Bartonella clarridgeiae, and R. felis) (Bitam et al., 2010), and also acts as the intermediate host of the tapeworm Dipylidium caninum (Guzman, 1984). Whilst the dog flea Ctenocephalides canis is climatically restricted to the temperate regions, Ctenocephalides orientis (Fig. 2C) is mainly distributed in tropical Asia (i.e. India and SEA) (Colella et al., 2020; Hii et al., 2015; Kernif et al., 2012). Indeed, recently acquired knowledge (Calvani et al., 2020; Colella et al., 2020; Lawrence et al., 2019) indicates that previous reports of C. canis parasitizing domestic dogs in SEA probably refer to C. orientis, due to their strong morphological similarity. The vector competence of C. orientis in transmitting pathogens remains unclear although this flea species has been found to carry some rickettsiae such as Rickettsia asembonensis and Rickettsia sp. genotype RF2125 (Nguyen et al., 2020; Phoosangwalthong et al., 2018).
Mosquitoes play an important role in the transmission of various pathogens to dogs, cats, and humans worldwide, including SEA. The occurrence of Dirofilaria spp. and Brugia spp. has been widely reported in this region, and mosquitoes of the genera Aedes, Armigeres, and Mansonia are responsible for the transmission of these filarial pathogens (Denham & McGreevy, 1977; Irwin & Jefferies, 2004).
3. Vector-borne pathogens of zoonotic concern
Rickettsia felis is an emerging bacterial pathogen, which can be found in mammalian hosts and arthropods worldwide, with C. felis acting as the main vector and reservoir for this pathogen (Legendre & Macaluso, 2017; Parola, 2011). More recently, dogs have been demonstrated as competent reservoir hosts of R. felis with the infection resulting mostly subclinical symptoms (Ng-Nguyen et al., 2020). Since the first human case of flea-borne spotted fever attributed to R. felis in Thai-Myanmar border (Parola et al., 2003a), several cases of R. felis infection in patients with non-specific febrile illness have been documented in SEA, including Thailand (Edouard et al., 2014), Laos (Dittrich et al., 2014), Vietnam (Le-Viet et al., 2019), and Indonesia (Mawuntu et al., 2020). This pathogen has been detected in C. felis from dogs and cats from Indonesia, Laos, Malaysia, the Philippines, Thailand, and Vietnam (Kernif et al., 2012; Nguyen et al., 2020b). Meanwhile, a study reported that 10.9% of 101 free-roaming owned dogs from Cambodia were molecularly positive for R. felis (Inpankaew et al., 2016).
Cats are the main reservoirs for B. henselae, B. clarridgeiae, and Bartonella koehlerae, which cause cat scratch disease and endocarditis in humans (Chomel et al., 2006). Of these, B. henselae and B. clarridgeiae were reported in cats and their fleas from Indonesia, Malaysia, the Philippines, Singapore, and Thailand with prevalences of up to 60% (Chomel et al., 1999; Marston et al., 1999; Maruyama et al., 2001; Mokhtar & Tay, 2011; Nasirudeen & Thong, 1999), whereas B. koehlerae was detected for the first time in SEA in cats and C. felis from Thailand (Assarasakorn et al., 2012). Human infection by B. henselae has been reported to cause endocarditis in Laos and Thailand (Noopetch et al., 2018; Rattanavong et al., 2014; Watt et al., 2014), and ocular neuroretinitis in Malaysia (Tan et al., 2017). Additionally, Bartonella vinsonii subsp. berkhoffii, another important agent of human endocarditis, was detected in cats and dogs from Thailand (Srisanyong et al., 2016; Suksawat et al., 2001). Several other zoonotic species and subspecies of Bartonella have been identified in dogs from Thailand including B. clarridgeiae, Bartonella elizabethae, Bartonella quintana, and B. vinsonii subsp. arupensis (Bai et al., 2010; Billeter et al., 2012), with the latter being also found in Thai patients (Bai et al., 2012). Recently, B. henselae was also found to infect dogs in the Philippines (Singer et al., 2020).
Lyme borreliosis by Borrelia burgdorferi (s.l.) is mostly prevalent in the temperate northern hemisphere (Lantos et al., 2014). These bacteria are transmitted to dogs and humans by tick species of the genus Ixodes, particularly Ixodes ricinus and Ixodes persulcatus in Europe and northern Asia, and Ixodes scapularis in North America (Dantas-Torres et al., 2012a; Jongejan & Uilenberg, 2004). The presence of B. burgdorferi (s.l.) has also been serologically and molecularly confirmed in dogs in Thailand (Sthitmatee et al., 2016). Recently, B. burgdorferi (s.l.) has been serologically diagnosed in dogs from Indonesia and the Philippines (Colella et al., 2020). Some unexplained seropositive results have also been reported in other non-endemic areas worldwide, which could also suggest the occurrence of rare, but possible cross-reaction (Azzag et al., 2015; Maggi & Krämer, 2019).
Leishmania infantum, the causative agent of canine leishmaniasis, is among the most important zoonotic VBP of dogs, which has been found in all continents, except Oceania (Dantas-Torres et al., 2012b). In the Old World, this parasite is transmitted by various species of phlebotomine sand flies within the genus Phlebotomus (Killick-Kendrick, 1990), and causes visceral and/or cutaneous leishmaniasis in dogs and humans (Dantas-Torres et al., 2012b). Canine leishmaniasis is endemic in many regions of the world, such as South America and the Mediterranean basin (Otranto & Dantas-Torres, 2013). In SEA, L. infantum has been serologically diagnosed in dogs in the Philippines and Vietnam (Colella et al., 2020), and the presence of L. infantum has also been molecularly confirmed in one patient in Thailand (Maharom et al., 2008). Even though SEA is not considered as a L. infantum-endemic area, a study showed a high seroprevalence (55.3%) in immigrant workers in Malaysia; however, none of the tested phlebotomine sand flies from the same survey was found positive for Leishmania spp. by PCR (Noor Azian et al., 2016), which raises some doubts about the origin of these infections.
Trypanosoma evansi is a protozoan transmitted by hematophagous flies of the genera Stomoxys and Tabanus, which has been found in various mammalian hosts including bovines, rodents, canines, and humans in tropical and subtropical regions (Aregawi et al., 2019). This parasite is of great veterinary concern due to its ability to cause severe illness in animals such as dogs and horses (Desquesnes et al., 2013). Many cases of canine trypanosomiasis have been reported in South America, Africa, Europe, and Asia (Defontis et al., 2012; Howes et al., 2011; Panigrahi et al., 2015; Rashid et al., 2014; Rjeibi et al., 2015). In SEA, some cases of canine trypanosomiasis have been reported in Malaysia and Thailand (Barameechaithanun et al., 2009; Rajamanickam et al., 1985). The presence of T. evansi has also been molecularly detected in a dog in Vietnam (Bui et al., 2020). Other than dogs, T. evansi is highly prevalent in cattle and water buffaloes in SEA (Desquesnes et al., 2009; Verloo et al., 2000). Notably, some cases of human infections with T. evansi have been reported, including one from Vietnam (Joshi et al., 2006; Powar et al., 2006; Van Vinh Chau et al., 2016), raising concerns about its zoonotic potential in endemic regions.
Lymphatic filariasis (LF), commonly known as elephantiasis, is one of the neglected tropical diseases, and it has been considered for a long time as endemic in SEA (Noordin et al., 2013). An estimated 15 million people in SEA are affected by LF (Sudomo et al., 2010). The disease is mainly due to the infection with Wuchereria bancrofti, Brugia malayi, and Brugia timori, which are transmitted by mosquitoes (World Health Organization, 2010). In particular, W. bancrofti is responsible for 90% LF cases, and the remaining are mostly due to B. malayi (World Health Organization, 2010). Domestic cats are recognized as reservoirs of the zoonotic nocturnal subperiodic form of B. malayi. The infection has been reported in cats with a prevalence ranging from 8.2% in Malaysia (Al-Abd et al., 2015) up to 28.3% in southern Thailand (Chansiri et al., 2002). The recent findings of B. malayi DNA in dogs from Thailand and Vietnam (Colella et al., 2020), and from other countries (e.g. Sri Lanka and India) in neighboring regions (Mallawarachchi et al., 2018; Manoj et al., 2020) highlight the importance of domestic dogs as potential reservoirs for this zoonotic filarial nematode in these countries. Brugia malayi is one of the main targets of the Global Programme for the Eliminate of Lymphatic Filariasis (GPELF), which aimed to eradicate this disease as a public health problem by 2020 through mass drug administration (MDA). Some countries such as Cambodia, Thailand, and Vietnam have already achieved the eradication of LF and others are still accomplishing this goal (World Health Organization, 2020). Mosquitoes of the genus Mansonia (e.g. Mansonia bonneae and Mansonia dives), which have a wide distribution in SEA, are recognized as the main vectors of this filarial nematode (Zielke et al., 1993). Brugia pahangi, a species closely related to B. malayi, was found in dogs and cats from Indonesia, Malaysia, and Thailand. Although this filarial nematode was not considered as infecting humans under natural conditions, the first cases of human filariasis caused by B. pahangi have been reported in Malaysia (Tan et al., 2011). Thereafter, B. pahangi was found causing ocular infection in a Malaysian patient, and the microfilariae of this filarial nematode were also found in her cat and in Armigeres subalbatus mosquitoes from surrounding areas (Muslim et al., 2013a). This mosquito species has been proven as vector of B. pahangi (Muslim et al., 2013b) along with Mansonia annulata and M. dives (Laing et al., 1960).
Other mosquito-borne filarial nematodes of zoonotic concern in SEA are D. immitis and Dirofilaria repens. These parasites are widely distributed, and can be found in many animal species, including humans (Dantas-Torres & Otranto, 2020; Otranto et al., 2013; Simón et al., 2012). Approximately, 70 mosquito species mainly from the genera Culex, Aedes, and Anopheles are considered as competent vectors of D. immitis and D. repens, causing animal and human heartworm and subcutaneous diseases, respectively (Eldridge & Edman, 2000). Among the mosquito vectors, Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus, which are commonly found in rural and urban areas of SEA, may be responsible for the transmission of these two Dirofilaria spp. (Tiawsirisup & Kaewthamasorn, 2007; Tiawsirisup & Nithiuthai, 2006). Dirofilaria immitis was reported in a human patient in Thailand with a pulmonary nodule at the right lower lobe (Sukpanichnant et al., 1998). Canine heartworm infection caused by D. immitis is endemic in SEA, where the prevalence reported in dogs ranges from 16% in Cambodia to 24% in Thailand (Inpankaew et al., 2016; Kamyingkird et al., 2017). Studies also reported the presence of D. immitis in cats in Indonesia, Malaysia, and Thailand with the higher infection prevalence (36%) recorded in southern Thailand (Kamyingkird et al., 2017). On the other hand, D. repens was also found in cats from those aforementioned countries with the highest prevalence of 12% in Malaysia (Al-Abd et al., 2015). This filarial nematode is endemic in dogs from Europe, where many cases of human subcutaneous/ocular infection have been diagnosed (Capelli et al., 2018; Otranto & Eberhard, 2011). In SEA, many cases of ocular infections with D. repens have been reported, including two cases in Thailand (Jariya & Sucharit, 1983; Pradatsundarasar, 1985), four in Malaysia (Rohela et al., 2009), and 11 in Vietnam (Dang et al., 2010; Van De et al., 2012). Additionally, a rare case of subcutaneous infection with D. repens on the posterior thoracic region was also reported in Vietnam (Le et al., 2015). Recently, a case of a Thai patient with subconjunctival dirofilariasis caused by Dirofilaria sp., closely related to the Dirofilaria sp. found in humans in Hongkong and India (To et al., 2012), has been documented (Sukudom et al., 2018). Moreover, two species/genotypes of D. repens-like filarial nematodes (referred to as Dirofilaria sp. “Thailand II” and Dirofilaria sp. “Thailand III”) were reported in cats from Thailand (Yilmaz et al., 2016, 2019), which indicates that the genetic diversity of filarial nematodes in SEA may be currently underestimated.
Thelazia callipaeda, also known as the “oriental eye-worm” was initially described in the former Soviet Union and in many countries in the Far East (Otranto et al., 2020). Zoophilic fruit flies of the genus Phortica act as intermediate hosts of this parasite in Europe (i.e. Phortica variegata) and in SEA (i.e. Phortica okadai) (Otranto et al., 2004). This nematode causes ocular infection in carnivores (e.g. dogs, cats, and foxes) and humans (Otranto et al., 2004). Human ocular infections have been reported in Indonesia, Thailand, and Vietnam (Bhaibulaya et al., 1970; Kosin et al., 1989; Van De et al., 2012; Viriyavejakul et al., 2012; Yospaiboon et al., 1989). The infection was also reported in dogs from Thailand (Bhaibulaya et al., 1970). However, none of the dogs and cats tested in a recent survey from SEA countries was found positive for T. callipaeda (Colella et al., 2020).
Dipylidium caninum is a tapeworm infecting dogs and cats worldwide, with their fleas (e.g. C. felis, C. canis, and C. orientis) and lice (e.g. Trichodectes canis) serving as intermediate hosts (Labuschagne et al., 2018). The infection in dogs and cats occurs by accidental ingestion of infected intermediate hosts (Guzman, 1984). Similarly, the infection in humans may occur through this route (García-Agudo et al., 2014; Sapp & Bradbury, 2020), and it has been recorded in at least 24 countries (Jiang et al., 2017). In SEA, since a human case was documented in an old study from the Philippines (Mendoza-Guazon & Abad, 1916), no other case has been reported, although some infections have been detected in neighboring countries such as India (Narasimham et al., 2013) and China (Jiang et al., 2017). The true incidence of D. caninum in humans seems to be underestimated considering the asymptomatic infection, and that examination for the presence of proglottids in faeces is seldom performed (Sapp & Bradbury, 2020). Conversely, the infection is commonly found in dogs and cats, especially in stray populations or those from rural areas. Some studies in Malaysia using fecal examination revealed a prevalence of D. caninum infection in rural dogs and stray cats reaching up 3.7% and 11.6%, respectively (Mohd Zain et al., 2013; Ngui et al., 2014). Interestingly, a recent molecular characterization of D. caninum confirmed the existence of two distinct genotypes (i.e. canine and feline genotypes), which are apparently host specific (Beugnet et al., 2018; Labuschagne et al., 2018). Additionally, C. felis and Felicola subrostratus collected from cats in Malaysia were also found to harbour DNA of the feline genotype of D. caninum (Labuschagne et al., 2018; Low et al., 2017).
4. Other VBP affecting dogs and cats
Other VBP of veterinary concern, which affect animal health and welfare, have also been reported in SEA. Some of them may also cause mortality in dogs as it is the case of E. canis, the causative agent of canine monocytic ehrlichiosis. This bacterium is widespread and considered as highly virulent to dogs in SEA (Colella et al., 2020; Niwetpathomwat et al., 2006). In the past, this tick-borne pathogen was responsible for the death of hundreds of US military dogs serving in Vietnam (Kelch, 1984). In a study conducted in Thailand, 33% of dogs infected by E. canis had fever and in 55% of them the body temperature higher than 40 °C (Niwetpathomwat et al., 2006). Another bacterial pathogen commonly reported in dogs in SEA is A. platys, the causative agent of canine cyclic thrombocytopenia, with a prevalence reaching up to 38.5% in stray dogs from Malaysia (Mohammed et al., 2017). This bacterium was also reported in a domestic cat from Thailand (Salakij et al., 2012).
Two species of Babesia (B. vogeli and Babesia gibsoni) have been reported in dogs from SEA (Inpankaew et al., 2016; Prakash et al., 2018b). These parasites may cause clinical conditions (e.g. lethargy, anemia, and thrombocytopenia) as reported for B. vogeli in dogs from the Philippines (Ybañez et al., 2017). Another protozoan commonly found in SEA is H. canis (Colella et al., 2020; Inpankaew et al., 2016), which is considered less virulent, although it can cause a range of clinical signs in dogs (Baneth et al., 2003).
Haemotropic mycoplasmas, also known as haemoplasmas, are widespread bacteria of cats and other carnivores that can be found all over the world (Barrs et al., 2010; Latrofa et al., 2020; Soto et al., 2017). The infection can induce clinical spectrum ranges from subclinical to haemolytic anemia and life-threatening conditions, particularly in immunocompromised hosts (Willi et al., 2007). Three haemoplasmas (i.e. Mycoplasma haemofelis, “Candidatus Mycoplasma haemominutum”, and “Candidatus Mycoplasma turicensis”) are mostly found infecting cats (Barker, 2019; Do et al., 2020), whereas Mycoplasma haemocanis and “Candidatus Mycoplasma haematoparvum” are commonly found in dogs (Huggins et al., 2019; Soto et al., 2017). The natural transmission routes of haemoplasmas in dogs and cats remain unclear, though the vector competence of R. sanguineus (s.l.) and C. felis to transmit M. haemocanis and M. haemofelis, respectively, has been considered (Senevtratna et al., 1973; Woods et al., 2005). Direct transmission by biting and blood transfusion have also been reported (Willi et al., 2007). In SEA, the infection with Mycoplasma spp. has received less attention, with few studies conducted so far. However, high prevalence of Mycoplasma spp. was reported in community dogs (40%) and cats (38%) from Thailand (Do et al., 2020; Huggins et al., 2019) as well as in free-roaming dogs (12.8%) from Cambodia (Inpankaew et al., 2016), suggesting the common occurrence of these bacteria in dogs and cats in SEA.
5. Managing zoonotic VBP
5.1. Diagnosis
Several limitations (e.g. relatively high costs of diagnostic tests, scarce laboratory infrastructures, and lack of diagnostic expertise) affect the ability of veterinarians to achieve reliable diagnoses of VBP of dogs and cats in SEA as well as other developing regions (Dantas-Torres et al., 2020; Otranto, 2015). Due to its relatively low cost and high sensitivity, the Knott's test is the most popular procedure for detecting microfilariae in SEA (Noordin et al., 2013). Although the presence of microfilariae can be microscopically identified in the bloodstream of infected animals (Panarese et al., 2020), their identification based on morphology and measurements may be troublesome. For example, microfilariae of B. malayi are around 220 μm in length and 5 μm in diameter, and those of B. pahangi are around 280 × 5 μm (Schacher, 1962; Taylor, 1960). Dirofilaria immitis microfilariae are 290–330 μm in length and 5–7 μm in diameter, whereas those of D. repens are slightly longer and wider (350–385 × 7–8 μm) (Simón et al., 2012). Moreover, false negative results may occur in cases of adult single-sex infection or low microfilaremia. Many studies have been conducted based on serological surveys by using point-of-care (POC) commercial kits to detect antigen of D. immitis females in serum or blood samples (Chelliah & Šlapeta, 2019; Colella et al., 2020; Sukhumavasi et al., 2012; Theis et al., 2008). However, no similar serological tests are available for the diagnosis of other filarial infections. Serological tests such as immunofluorescence antibody, western immunoblot, POC tests (e.g. SNAP 4Dx Plus and SNAP Leishmania) have also been applied to detect the presence of antibodies to several pathogens (i.e. Anaplasma spp., Ehrlichia spp., Bartonella spp., B. burgdorferi, and Leishmania spp.) (Chomel et al., 1999; Colella et al., 2020; Suksawat et al., 2001). Additionally, the misinterpretation of cytology may also lead to the misdiagnosis of some microorganisms, e.g. reports of “Babesia canis” in dogs in SEA probably refer to B. vogeli (Petney et al., 2019). Therefore, the use of molecular assays for pathogen detection is recommended, as they are faster and more accurate. A study in Thailand revealed a significantly higher prevalence of D. immitis infection in dogs and cats by using conventional PCR (cPCR) compared to microscopic examination (Kamyingkird et al., 2017). A real-time fluorescence resonance energy transfer PCR assay has also been used to diagnose the infection with B. malayi and B. pahangi (Thanchomnang et al., 2010). More recently, a real-time PCR followed by high resolution melting analysis has been developed for the detection of multiple filarial nematodes (i.e. Acanthocheilonema reconditum, B. malayi, B. pahangi, D. immitis, and D. repens) in cats (Wongkamchai et al., 2014) and also in mosquito vectors (Thanchomnang et al., 2013). Many molecular surveys using cPCR and real-time PCR to detect the presence of bacterial and protozoal pathogens in dogs, cats, and their associated arthropods have been conducted (Colella et al., 2020; Inpankaew et al., 2016; Kernif et al., 2012; Singer et al., 2020). Recently, next-generation sequencing, which provides a powerful tool to diagnose multiple pathogens with higher sensitivity compared to cPCR, has also been applied for the detection of canine VBP in Thailand (Huggins et al., 2019). However, considering the highly required laboratory infrastructures, expertise, and cost per sample tested for molecular assays (Momčilović et al., 2019), the application of molecular techniques for diagnosing VBP in dogs and cats as well as their associated vectors has been limited in some countries such as Cambodia, Laos, Myanmar, and Timor-Leste. As such, the prevalence and clinical importance of many VBP in dogs and cats are probably largely underestimated in SEA.
5.2. Prevention and control
As for other diseases, VBD prevention is always better than cure (Dantas-Torres & Otranto, 2016). The prevention of VBD is strongly linked to the control of their arthropod vectors (Otranto, 2018). The control of arthropod vectors such as ticks, fleas, and mosquitoes should be performed simultaneously by using an integrated approach focusing on animals and the environment (Otranto & Wall, 2008). Many acaricides and insecticides in several formulations (e.g. topical sprays, spot-on, baths, dusting powders, and collars), which have a long-lasting effect, and are safe for pets and their owners, are commercially available in SEA. For instance, the combination containing 10% imidacloprid and 50% permethrin can prevent dogs from Ae. aegypti mosquito bites for up to 3–4 weeks (Tiawsirisup et al., 2007). The use of afoxolaner can provide an effective treatment (> 96%) against adult R. sanguineus (s.l.) in dogs in the cases of heavy infestation (Tinkruejeen et al., 2019). Nevertheless, the possible emergence of resistant strains of pathogens and their arthropod vectors due to the indiscriminate use of insecticides, acaricides, and antibiotics should be carefully considered (Otranto et al., 2009). Among the many herbal extracts used as ectoparasites repellents, more than 2,300 plant species were found to have potential mosquito repellent properties across SEA (Tisgratog et al., 2016). For instance, many studies in Thailand revealed the high repellency (up to 8 h) of essential oils extracted from Angelica sinensis (dong quai), Psidium guajava (guava), Curcuma longa (turmeric), and Piper nigrum (black pepper) against some mosquito species, such as Ae. aegypti, Ae. albopictus, and Cx. quinquefasciatus (Champakaew et al., 2015; Tawatsin et al., 2006). The essential oil of Cymbopogon citratus (lemon grass) has been widely used in many countries in SEA as a cheap and effective mosquito repellent (Shah et al., 2011). Additionally, some biological control agents (i.e. Mesocyclops and Wolbachia) against Aedes spp. have also been applied. The application of Mesocyclops for eradication of Ae. aegypti in some provinces with high incidence of dengue in Vietnam have achieved a great accomplishment with approximately 90% of Ae. aegypti larval populations reduced after one year of intervention (Vu et al., 2005). Along with the MDA, which has been given to people living in LF endemic areas, the control programme of filarial nematodes among reservoir host populations (i.e. domestic dogs and cats) should be considered as one of the comprehensive strategies to archive the target of the GPELF. The use of doxycycline alone or in combination with ivermectin for treatment of B. malayi infection in cats has given a good eradication effect to both microfilariae and adult worms (Khowawisetsut et al., 2017). Recently, guidelines for the diagnosis, preventions and treatments of parasitic diseases in companion dogs and cats in the tropics have been prepared by the Tropical Council of Companion Animal Parasites (Dantas-Torres et al., 2020). The guidelines are freely released in multiple languages including English, Bahasa Malaysia, Thai, and Vietnamese (https://www.troccap.com), which are very useful for local veterinarians and pet owners to improve the awareness, prevention and control of VBD.
6. Conclusions and research needs
The updated data discussed in this review illustrate a general picture of VBD affecting dogs and cats in SEA, which represent an important issue to animal and public health. Overall, zoonotic VBP, such as R. felis, B. henselae, and D. repens, are of concern to human health in this region. Despite the recently acquired scientific knowledge, many scientific gaps still persist about the eco-epidemiology of the zoonotic VBP, which limit our current understanding and our capability to control them. For instance, although many human cases of dirofilariasis by D. repens have been reported (Rohela et al., 2009; Van De et al., 2012), the source of infection as well as the species of mosquito vectors of this nematode in SEA remains unclear. Therefore, the zoonotic transmission cycle of D. repens in SEA deserves further investigations. In spite of the acquired data for some countries such as Malaysia and Thailand (Colella et al., 2020; Huggins et al., 2019; Koh et al., 2016; Low et al., 2018; Wongkamchai et al., 2014), data regarding the distribution of the zoonotic VBP in other countries (e.g. Cambodia, Laos, Myanmar, and Timor-Leste) is limited or virtually inexistent. On the other hand, the rapidly changing of environment (e.g. climate change, land use change, and urbanization) in SEA may also alter the distribution and abundance of vectors and VBD (Dantas-Torres, 2015; Lim & Vythilingam, 2013). Therefore, further epidemiological surveillance as well as studies on the impact of those environmental factors to the distribution of the zoonotic VBP in SEA are advocated. Finally, a stronger collaboration between governments, commercial companies, scientists, and medical and veterinary communities should be implemented for a better management of VBD in SEA. The effective control measures of canine and feline VBD as well as their arthropod vectors should be performed simultaneously for a better prevention of zoonotic infections.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Authors’ contributions
DO conceived the study. VLN conducted the literature screening, analyzed the data, and wrote the first draft of the manuscript. FDT and DO critically revised the manuscript. All authors read and approved the final manuscript.
Funding
This study received no specific funding.
Acknowledgement
The authors thank Tran Nam Trieu (Fig. 2A) and Do Minh Hoan (Fig. 2B) for kindly sharing their photos.
References
- Al-Abd N.M., Nor Z.M., Kassim M., Mansor M., Al-Adhroey A.H., Ngui R., Sivanandam S. Prevalence of filarial parasites in domestic and stray cats in Selangor State, Malaysia. Asian Pac. J. Trop. Med. 2015;8:705–709. doi: 10.1016/j.apjtm.2015.07.034. [DOI] [PubMed] [Google Scholar]
- Aregawi W.G., Agga G.E., Abdi R.D., Büscher P. Systematic review and meta-analysis on the global distribution, host range, and prevalence of Trypanosoma evansi. Parasit. Vectors. 2019;12:1–25. doi: 10.1186/s13071-019-3311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assarasakorn S., Veir J.K., Hawley J.R., Brewer M.M., Morris A.K., Hill A.E., Lappin M.R. Prevalence of Bartonella species, hemoplasmas, and Rickettsia felis DNA in blood and fleas of cats in Bangkok, Thailand. Res. Vet. Sci. 2012;93:1213–1216. doi: 10.1016/j.rvsc.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Aung S.T. MSc Thesis, University of Veterinary Science, Nay Pyi Taw; Myanmar: 2014. Prevalence and associated risk factors of Dirofilaria immitis in dogs within Nay Pyi Taw area. [Google Scholar]
- Azzag N., Petit E., Gandoin C., Bouillin C., Ghalmi F., Haddad N., Boulouis H.J. Prevalence of select vector-borne pathogens in stray and client-owned dogs from Algiers. Comp. Immunol. Microbiol. Infect. Dis. 2015;38:1–7. doi: 10.1016/j.cimid.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Bai Y., Kosoy M.Y., Boonmar S., Sawatwong P., Sangmaneedet S., Peruski L.F. Enrichment culture and molecular identification of diverse Bartonella species in stray dogs. Vet. Microbiol. 2010;146:314–319. doi: 10.1016/j.vetmic.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Bai Y., Kosoy M.Y., Diaz M.H., Winchell J., Baggett H., Maloney S.A., et al. Bartonella vinsonii subsp. arupensis in humans, Thailand. Emerg. Infect. Dis. 2012;18:989–991. doi: 10.3201/eid1806.111750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baneth G., Mathew J.S., Shkap V., Macintire D.K., Barta J.R., Ewing S.A. Canine hepatozoonosis: Two disease syndromes caused by separate Hepatozoon spp. Trends Parasitol. 2003;19:27–31. doi: 10.1016/s1471-4922(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Barameechaithanun E., Suwannasaeng P., Boonbal N., Pattanee S., Hoisang S. Treatment of trypanosomiasis in dog. J. Mahanakorn Vet. Med. 2009;4:51–60. [Google Scholar]
- Barker E.N. Update on feline hemoplasmosis. Vet. Clin. North Am. Small Anim. Pract. 2019;49:733–743. doi: 10.1016/j.cvsm.2019.02.009. [DOI] [PubMed] [Google Scholar]
- Barrs V., Beatty J., Wilson B., Evans N., Gowan R., Baral R., Lingard A., Perkovic G., Hawley J., Lappin M. Prevalence of Bartonella species, Rickettsia felis, haemoplasmas and the Ehrlichia group in the blood of cats and fleas in eastern Australia. Aust. Vet. J. 2010;88:160–165. doi: 10.1111/j.1751-0813.2010.00569.x. [DOI] [PubMed] [Google Scholar]
- Beugnet F., Labuschagne M., De Vos C., Crafford D., Fourie J. Analysis of Dipylidium caninum tapeworms from dogs and cats, or their respective fleas - Part 2. Distinct canine and feline host association with two different Dipylidium caninum genotypes. Parasite. 2018;25:31. doi: 10.1051/parasite/2018029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaibulaya M., Prasertsilpa S., Vajrasthira S. Thelazia callipaeda Railliet and Henry, 1910, in man and dog in Thailand. Am. J. Trop. Med. Hyg. 1970;19:476–479. doi: 10.4269/ajtmh.1970.19.476. [DOI] [PubMed] [Google Scholar]
- Billeter S.A., Sangmaneedet S., Kosakewich R.C., Kosoy M.Y. Bartonella species in dogs and their ectoparasites from Khon Kaen province, Thailand. Southeast Asian J. Trop. Med. Public Health. 2012;43:1186–1192. [PubMed] [Google Scholar]
- Bitam I., Dittmar K., Parola P., Whiting M.F., Raoult D. Fleas and flea-borne diseases. Int. J. Infect. Dis. 2010;14:667–676. doi: 10.1016/j.ijid.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Bui K.L., Duong D.H., Bui T.A.D., Nguyen V.-L., Do T., Le T.L.A., Tran K.T. A case of Trypanosoma evansi in a German shepherd dog in Vietnam. Parasitol. Int., 2020;80 doi: 10.1016/j.parint.2020.102198. [DOI] [PubMed] [Google Scholar]
- Calvani N.E.D., Bell L., Carney A., De La Fuente C., Stragliotto T., Tunstall M., Šlapeta J. The molecular identity of fleas (Siphonaptera) carrying Rickettsia felis, Bartonella clarridgeiae and Bartonella rochalimae from dogs and cats in Northern Laos. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelli G., Genchi C., Baneth G., Bourdeau P., Brianti E., Cardoso L., et al. Recent advances on Dirofilaria repens in dogs and humans in Europe. Parasit. Vectors. 2018;11:663. doi: 10.1186/s13071-018-3205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champakaew D., Junkum A., Chaithong U., Jitpakdi A., Riyong D., Sanghong R., et al. Angelica sinensis (Umbelliferae) with proven repellent properties against Aedes aegypti, the primary dengue fever vector in Thailand. Parasitol. Res. 2015;114:2187–2198. doi: 10.1007/s00436-015-4409-z. [DOI] [PubMed] [Google Scholar]
- Chansiri K., Tejangkura T., Kwaosak P., Sarataphan N., Phantana S., Sukhumsirichart W. PCR based method for identification of zoonostic Brugia malayi microfilariae in domestic cats. Mol. Cell. Probes. 2002;16:129–135. doi: 10.1006/mcpr.2001.0402. [DOI] [PubMed] [Google Scholar]
- Chelliah M.K., Šlapeta J. The prevalence and trends of canine heartworm (Dirofilaria immitis) in Kuala Lumpur, Malaysia (1970–2018) Vet. Parasitol. Reg. Stud. Reports. 2019;16:100272. doi: 10.1016/j.vprsr.2019.100272. [DOI] [PubMed] [Google Scholar]
- Chien N.T.H., Nguyen T.L., Bui K.L., Nguyen T. Van, Le T.H. Anaplasma marginale and A. platys characterized from dairy and indigenous cattle and dogs in northern Vietnam. Korean J. Parasitol. 2019;57:43–48. doi: 10.3347/kjp.2019.57.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomel B.B., Boulouis H.J., Maruyama S., Breitschwerdt E.B. Bartonella spp. in pets and effect on human health. Emerg. Infect. Dis. 2006;12:389–394. doi: 10.3201/eid1203.050931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomel B.B., Carlos E.T., Kasten R.W., Yamamoto K., Chang C.C., Carlos R.S., et al. Bartonella henselae and Bartonella clarridgeiae infection in domestic cats from the Philippines. Am. J. Trop. Med. Hyg. 1999;60:593–597. doi: 10.4269/ajtmh.1999.60.593. [DOI] [PubMed] [Google Scholar]
- Colella V., Nguyen V.L., Tan D.Y., Lu N., Fang F., Zhijuan Y., et al. Zoonotic vectorborne pathogens and ectoparasites of dogs and cats in Eastern and Southeast Asia. Emerg. Infect. Dis. 2020;26:1221–1233. doi: 10.3201/eid2606.191832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corales J.M., Viloria V.V., Venturina V.M., Mingala C.N. The prevalence of Ehrlichia canis, Anaplasma platys and Babesia spp. in dogs in Nueva Ecija, Philippines based on multiplex polymerase chain reaction (mPCR) assay. Ann. Parasitol. 2014;60:267–272. [PubMed] [Google Scholar]
- Dang T.C.T., Nguyen T.H., Do T.D., Uga S., Morishima Y., Sugiyama H., Yamasaki H. A human case of subcutaneous dirofilariasis caused by Dirofilaria repens in Vietnam: Histologic and molecular confirmation. Parasitol. Res. 2010;107:1003–1007. doi: 10.1007/s00436-010-1961-4. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F. Climate change, biodiversity, ticks and tick-borne diseases: The butterfly effect. Int. J. Parasitol. Parasites Wildl. 2015;4:452–461. doi: 10.1016/j.ijppaw.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas-Torres F., Chomel B.B., Otranto D. Ticks and tick-borne diseases: A one health perspective. Trends Parasitol. 2012;28:437–446. doi: 10.1016/j.pt.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F., Ketzis J., Mihalca A.D., Baneth G., Otranto D., Tort G.P., et al. TroCCAP recommendations for the diagnosis, prevention and treatment of parasitic infections in dogs and cats in the tropics. Vet. Parasitol. 2020;283:109167. doi: 10.1016/j.vetpar.2020.109167. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F., Latrofa M.S., Annoscia G., Giannelli A., Parisi A., Otranto D. Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the New and Old Worlds. Parasit. Vectors. 2013;6:213. doi: 10.1186/1756-3305-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas-Torres F., Otranto D. Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet. Parasitol. 2015;208:9–13. doi: 10.1016/j.vetpar.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F., Otranto D. Best practices for preventing vector-borne diseases in dogs and humans. Trends Parasitol. 2016;32:43–55. doi: 10.1016/j.pt.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F., Otranto D. Overview on Dirofilaria immitis in the Americas, with notes on other filarial worms infecting dogs. Vet. Parasitol. 2020;282:109113. doi: 10.1016/j.vetpar.2020.109113. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F., Solano-Gallego L., Baneth G., Ribeiro V.M., de Paiva-Cavalcanti M., Otranto D. Canine leishmaniosis in the old and new worlds: Unveiled similarities and differences. Trends Parasitol. 2012;28:531–538. doi: 10.1016/j.pt.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Defontis M., Richartz J., Engelmann N., Bauer C., Schwierk V.M., Büscher P., Moritz A. Canine Trypanosoma evansi infection introduced into Germany. Vet. Clin. Pathol. 2012;41:369–374. doi: 10.1111/j.1939-165X.2012.00454.x. [DOI] [PubMed] [Google Scholar]
- Denham D.A., McGreevy P.B. Brugian filariasis: Epidemiological and experimental studies. Adv. Parasitol. 1977;15:243–309. doi: 10.1016/s0065-308x(08)60530-8. [DOI] [PubMed] [Google Scholar]
- Desquesnes M., Holzmuller P., Lai D.-H., Dargantes A., Lun Z.-R., Jittaplapong S. Trypanosoma evansi and surra: A review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. Biomed Res. Int. 2013;2013:194176. doi: 10.1155/2013/194176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desquesnes M., Kamyingkird K., Pruvot M., Kengradomkij C., Bossard G., Sarataphan N., Jittapalapong S. Antibody-ELISA for Trypanosoma evansi: Application in a serological survey of dairy cattle, Thailand, and validation of a locally produced antigen. Prev. Vet. Med. 2009;90:233–241. doi: 10.1016/j.prevetmed.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Dittrich S., Phommasone K., Anantatat T., Panyanivong P., Slesak G., Blacksell S.D., et al. Rickettsia felis infections and comorbid conditions, Laos, 2003–2011. Emerg. Infect. Dis. 2014;20:1402–1404. doi: 10.3201/eid2008.131308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do T., Kamyingkird K., Bui L.K., Inpankaew T. Genetic characterization and risk factors for feline hemoplasma infection in semi-domesticated cats in Bangkok, Thailand. Vet. World. 2020;13:975–980. doi: 10.14202/vetworld.2020.975-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durden L.A., Merker S., Beati L. The tick fauna of Sulawesi, Indonesia (Acari: Ixodoidea: Argasidae and ixodidae) Exp. Appl. Acarol. 2008;45:85–110. doi: 10.1007/s10493-008-9144-z. [DOI] [PubMed] [Google Scholar]
- Edouard S., Bhengsri S., Dowell S.F., Watt G., Parola P., Raoult D. Two human cases of Rickettsia felis infection, Thailand. Emerg. Infect. Dis. 2014;20:1780–1781. doi: 10.3201/eid2010.140905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge B.F., Edman J.D. Medical entomology. Springer; Netherlands: 2000. Introduction to medical entomology; pp. 1–12. [DOI] [Google Scholar]
- Erawan I., Tjahajati I., Nurcahyo W., Asmara W. Prevalence and risk factor of the Dirofilaria immitis infection in dogs slaughtered in Daerah Istimewa Yogyakarta. J. Vet. 2018;18:541–546. doi: 10.19087/jveteriner.2017.18.4.541. [DOI] [Google Scholar]
- Faizal M.D., Haryanto A., Tjahajati I. Diagnosis and molecular characterization of Anaplasma platys in dog patients in Yogyakarta area, Indonesia. Indones. J. Biotechnol. 2019;24:43–50. doi: 10.22146/ijbiotech.42750. [DOI] [Google Scholar]
- Foongladda S., Inthawong D., Kositanont U., Gaywee J. Rickettsia, Ehrlichia, Anaplasma, and Bartonella in ticks and fleas from dogs and cats in Bangkok. Vector Borne Zoonotic Dis. 2011;11:1335–1341. doi: 10.1089/vbz.2010.0174. [DOI] [PubMed] [Google Scholar]
- Fukugawa N. Theoretical framework for innovation policy in ASEAN. ERIA Res. Proj. Rep. 2018:23–60. [Google Scholar]
- Galay R.L., Manalo A.A.L., Dolores S.L.D., Aguilar I.P.M., Sandalo K.A.C., Cruz K.B., et al. Molecular detection of tick-borne pathogens in canine population and Rhipicephalus sanguineus (sensu lato) ticks from southern Metro Manila and Laguna, Philippines. Parasit. Vectors. 2018;11:643. doi: 10.1186/s13071-018-3192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Agudo L., García-Martos P., Rodríguez-Iglesias M. Dipylidium caninum infection in an infant: A rare case report and literature review. Asian Pac. J. Trop. Biomed. 2014;4:S565–S567. https://doi.org/10.12980/APJTB.4.2014APJTB-2014-0034. [Google Scholar]
- Guzman R.F. A survey of cats and dogs for fleas: With particular reference to their role as intermediate hosts of Dipylidium caninum. N. Z. Vet. J. 1984;32:71–73. doi: 10.1080/00480169.1984.35067. [DOI] [PubMed] [Google Scholar]
- Hassan U.L., Dhaliwal G.K., Watanabe M., Ong B.L., Tay S.T. Feline bartonellosis associated with some clinico-pathological conditions in a veterinary hospital in Selangor, Malaysia. Trop. Biomed. 2017;34:174–179. [PubMed] [Google Scholar]
- Hii S.F., Lawrence A.L., Cuttell L., Tynas R., Abd Rani P.A.M., Šlapeta J., Traub R.J. Evidence for a specific host-endosymbiont relationship between “Rickettsia sp. genotype RF2125” and Ctenocephalides felis orientis infesting dogs in India. Parasit. Vectors. 2015;8:169. doi: 10.1186/s13071-015-0781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Bottazzi M.E., Strych U., Chang L.Y., Lim Y.A.L., Goodenow M.M., AbuBakar S. Neglected tropical diseases among the association of Southeast Asian nations (ASEAN): Overview and update. PLoS Negl. Trop. Dis. 2015;9:1–16. doi: 10.1371/journal.pntd.0003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes F., Silva A., Athayde C., Costa M., Corrêa M.M.B., Tavares K., et al. A new therapeutic protocol for dogs infected with Trypanosoma evansi. Acta Sci. Vet. 2011;39:988. [Google Scholar]
- Huggins L.G., Koehler A.V., Ng-Nguyen D., Wilcox S., Schunack B., Inpankaew T., Traub R.J. Assessment of a metabarcoding approach for the characterisation of vector-borne bacteria in canines from Bangkok, Thailand. Parasit. Vectors. 2019;12:394. doi: 10.1186/s13071-019-3651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inpankaew T., Hii S.F., Chimnoi W., Traub R.J. Canine vector-borne pathogens in semi-domesticated dogs residing in northern Cambodia. Parasit. Vectors. 2016;9:253. doi: 10.1186/s13071-016-1552-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin P.J., Jefferies R. Arthropod-transmitted diseases of companion animals in Southeast Asia. Trends Parasitol. 2004;20:27–34. doi: 10.1016/j.pt.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Jariya P., Sucharit S. Dirofilaria repens from the eyelid of a woman in Thailand. Am. J. Trop. Med. Hyg. 1983;32:1456–1457. doi: 10.4269/ajtmh.1983.32.1456. [DOI] [PubMed] [Google Scholar]
- Jiang P., Zhang X., Liu R.D., Wang Z.Q., Cui J. A human case of zoonotic dog tapeworm, Dipylidium caninum (Eucestoda: Dilepidiidae), in China. Korean J. Parasitol. 2017;55:61–64. doi: 10.3347/kjp.2017.55.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jittapalapong S., Inparnkaew T., Pinyopanuwat N., Kengradomkij C., Sangvaranond A., Wongnakphet S. Gastrointestinal parasites of stray cats in Bangkok Metropolitan areas, Thailand. Kasetsart J. Nat. Sci. 2007;41:69–73. [Google Scholar]
- Jittapalapong S., Rungphisutthipongse O., Maruyama S., Schaefer J.J., Stich R.W. Detection of Hepatozoon canis in stray dogs and cats in Bangkok, Thailand. Ann. N. Y. Acad. Sci. 2006;1081:479–488. doi: 10.1196/annals.1373.071. [DOI] [PubMed] [Google Scholar]
- Jongejan F., Uilenberg G. The global importance of ticks. Parasitology. 2004;129:S3–S14. doi: 10.1017/S0031182004005967. [DOI] [PubMed] [Google Scholar]
- Joshi P.P., Chaudhari A., Shegokar V.R., Powar R.M., Dani V.S., Somalwar A.M., et al. Treatment and follow-up of the first case of human trypanosomiasis caused by Trypanosoma evansi in India. Trans. R. Soc. Trop. Med. Hyg. 2006;100:989–991. doi: 10.1016/j.trstmh.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Kaewmongkol G., Lukkana N., Yangtara S., Kaewmongkol S., Thengchaisri N., Sirinarumitr T., et al. Association of Ehrlichia canis, hemotropic Mycoplasma spp. and Anaplasma platys and severe anemia in dogs in Thailand. Vet. Microbiol. 2017;201:195–200. doi: 10.1016/j.vetmic.2017.01.022. [DOI] [PubMed] [Google Scholar]
- Kamyingkird K., Junsiri W., Chimnoi W., Kengradomkij C., Saengow S., Sangchuto K., et al. Prevalence and risk factors associated with Dirofilaria immitis infection in dogs and cats in Songkhla and Satun provinces, Thailand. Agric. Nat. Resour. 2017;51:299–302. doi: 10.1016/j.anres.2017.05.003. [DOI] [Google Scholar]
- Kelch W.J. The canine ehrlichiosis (tropical canine pancytopenia) epizootic in Vietnam and its implications for the veterinary care of military working dogs. Mil. Med. 1984;149:327–331. doi: 10.1093/milmed/149.6.327. [DOI] [PubMed] [Google Scholar]
- Kernif T., Socolovschi C., Wells K., Lakim M.B., Inthalad S., Slesak G., et al. Bartonella and Rickettsia in arthropods from the Lao PDR and from Borneo, Malaysia. Comp. Immunol. Microbiol. Infect. Dis. 2012;35:51–57. doi: 10.1016/j.cimid.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khowawisetsut L., Sarasombath P.T., Thammapalo S., Loymek S., Korbarsa T., Nochote H., et al. Therapeutic trial of doxycyclin plus ivermectin for the treatment of Brugia malayi naturally infected cats. Vet. Parasitol. 2017;245:42–47. doi: 10.1016/j.vetpar.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Killick-Kendrick R. Phlebotomine vectors of the leishmaniases: A review. Med. Vet. Entomol. 1990;4:1–24. doi: 10.1111/j.1365-2915.1990.tb00255.x. [DOI] [PubMed] [Google Scholar]
- Koh F.X., Panchadcharam C., Tay S.T. Vector-borne diseases in stray dogs in peninsular Malaysia and molecular detection of Anaplasma and Ehrlichia spp. from Rhipicephalus sanguineus (Acari: Ixodidae) ticks. J. Med. Entomol. 2016 doi: 10.1093/jme/tjv153. [DOI] [PubMed] [Google Scholar]
- Kolonin G. Review of the ixodid tick fauna (Acari: Ixodidae) of Vietnam. J. Med. Entomol. 1995;32:276–282. doi: 10.1093/JMEDENT/32.3.276. [DOI] [PubMed] [Google Scholar]
- Kosin E., Kosman M.L., Depary A.A. First case of human thelaziasis in Indonesia. Southeast Asian J. Trop. Med. Public Health. 1989;20:233–236. [PubMed] [Google Scholar]
- Labuschagne M., Beugnet F., Rehbein S., Guillot J., Fourie J., Crafford D. Analysis of Dipylidium caninum tapeworms from dogs and cats, or their respective fleas - Part 1. Molecular characterization of Dipylidium caninum: Genetic analysis supporting two distinct species adapted to dogs and cats. Parasite. 2018;25:30. doi: 10.1051/parasite/2018028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing A.B.G., Edeson J.F.B., Wharton R.H. Studies on filariasis in Malaya: The vertebrate hosts of Brugia malayi and B. pahangi. Ann. Trop. Med. Parasitol. 1960;54:92–99. doi: 10.1080/00034983.1960.11685961. [DOI] [PubMed] [Google Scholar]
- Lantos P.M., Auwaerter P.G., Wormser G.P. A systematic review of Borrelia burgdorferi morphologic variants does not support a role in chronic Lyme disease. Clin. Infect. Dis. 2014;58:663–671. doi: 10.1093/cid/cit810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latrofa M.S., Dantas-Torres F., Giannelli A., Otranto D. Molecular detection of tick-borne pathogens in Rhipicephalus sanguineus group ticks. Ticks Tick. Borne. Dis. 2014;5:943–946. doi: 10.1016/j.ttbdis.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Latrofa M.S., Iatta R., Toniolo F., Furlanello T., Ravagnan S., Capelli G., et al. A molecular survey of vector-borne pathogens and haemoplasmas in owned cats across Italy. Parasit. Vectors. 2020;13:116. doi: 10.1186/s13071-020-3990-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.F., Dolah R.N., Mohammed K., Watanabe M., Abdul Rani P. Canine vector borne diseases of zoonotic concern in three dog shelters in peninsular Malaysia: The importance of preventive measures. Trop. Biomed. 2017;34:72–79. [PubMed] [Google Scholar]
- Lawrence A.L., Webb C.E., Clark N.J., Halajian A., Mihalca A.D., Miret J., et al. Out-of-Africa, human-mediated dispersal of the common cat flea, Ctenocephalides felis: The hitchhiker's guide to world domination. Int. J. Parasitol. 2019;49:321–336. doi: 10.1016/j.ijpara.2019.01.001. https://doi.org10.1016/j.ijpara.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Le-Viet N., Le V.N., Chung H., Phan D.T., Phan Q.D., Cao T. Van, et al. Prospective case-control analysis of the aetiologies of acute undifferentiated fever in Vietnam. Emerg. Microbes Infect. 2019;8:339–352. doi: 10.1080/22221751.2019.1580539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre K.P., Macaluso K.R. Rickettsia felis: A review of transmission mechanisms of an emerging pathogen. Trop. Med. Infect. Dis. 2017;2:64. doi: 10.3390/tropicalmed2040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T.A., Vi T.T., Nguyen K.L., Le T.H. A rare human case of Dirofilaria repens infection in the subcutaneous posterior thorax with molecular identification. Korean J. Parasitol. 2015;53:329–333. doi: 10.3347/kjp.2015.53.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y.A.L., Vythilingam I. In: Parasites and Their vectors: A special focus on Southeast Asia. Lim Y.A.L., Vythilingam I., editors. Springer-Verlag; Wien: 2013. Southeast Asia: Hotspot for parasitic infections; pp. 1–3. [DOI] [Google Scholar]
- Liu M., Ruttayaporn N., Saechan V., Jirapattharasate C., Vudriko P., Moumouni P.F.A., et al. Molecular survey of canine vector-borne diseases in stray dogs in Thailand. Parasitol. Int. 2016;65:357–361. doi: 10.1016/j.parint.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Low V.L., Prakash B.K., Lim Y.A.L., Tan T.K., Vinnie-Siow W.Y., Sofian-Azirun M., AbuBakar S. Detection of Anaplasmataceae agents and co-infection with other tick-borne protozoa in dogs and Rhipicephalus sanguineus sensu lato ticks. Exp. Appl. Acarol. 2018;75:429–435. doi: 10.1007/s10493-018-0280-9. [DOI] [PubMed] [Google Scholar]
- Low V.L., Prakash B.K., Tan T.K., Sofian-Azirun M., Anwar F.H.K., Vinnie-Siow W.Y., AbuBakar S. Pathogens in ectoparasites from free-ranging animals: Infection with Rickettsia asembonensis in ticks, and a potentially new species of Dipylidium in fleas and lice. Vet. Parasitol. 2017;245:102–105. doi: 10.1016/j.vetpar.2017.08.015. [DOI] [PubMed] [Google Scholar]
- Low V.L., Tan T.K., Khoo J.J., Lim F.S., AbuBakar S. An overview of rickettsiae in Southeast Asia: Vector-animal-human interface. Acta Trop. 2020;202:105282. doi: 10.1016/j.actatropica.2019.105282. [DOI] [PubMed] [Google Scholar]
- Maggi R.G., Krämer F. A review on the occurrence of companion vector-borne diseases in pet animals in Latin America. Parasit. Vectors. 2019;12:145. doi: 10.1186/s13071-019-3407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharom P., Siripattanapipong S., Mungthin M., Naaglor T., Sukkawee R., Pudkorn R., et al. Visceral leishmaniasis caused by Leishmania infantum in Thailand. Southeast Asian J. Trop. Med. Public Health. 2008;39:988–990. [PubMed] [Google Scholar]
- Mak J.W., Yen P.K., Lim K.C., Ramiah N. Zoonotic implications of cats and dogs in filarial transmission in Peninsular Malaysia. Trop. Geogr. Med. 1980;32:259–264. [PubMed] [Google Scholar]
- Mallawarachchi C.H., Chandrasena N.T.G.A., Wickramasinghe S., Premaratna R., Gunawardane N.Y.I.S., Mallawarachchi N.S.M.S.M., de Silva N.R. A preliminary survey of filarial parasites in dogs and cats in Sri Lanka. PLoS One. 2018;13 doi: 10.1371/journal.pone.0206633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoj R.R.S., Iatta R., Latrofa M.S., Capozzi L., Raman M., Colella V., Otranto D. Canine vector-borne pathogens from dogs and ticks from Tamil Nadu, India. Acta Trop. 2020;203:105308. doi: 10.1016/j.actatropica.2019.105308. [DOI] [PubMed] [Google Scholar]
- Marston E.L., Finkel B., Regnery R.L., Winoto I.L., Graham R.R., Wignal S., et al. Prevalence of Bartonella henselae and Bartonella clarridgeiae in an urban Indonesian cat population. Clin. Diagn. Lab. Immunol. 1999;6:41–44. doi: 10.1128/cdli.6.1.41-44.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama S., Sakai T., Morita Y., Tanaka S., Kabeya H., Boonmar S., et al. Prevalence of Bartonella species and 16S rRNA gene types of Bartonella henselae from domestic cats in Thailand. Am. J. Trop. Med. Hyg. 2001;65:783–787. doi: 10.4269/ajtmh.2001.65.783. [DOI] [PubMed] [Google Scholar]
- Mawuntu A.H.P., Johar E., Anggraeni R., Feliana F., Bernadus J.B.B., Safari D., et al. Rickettsia felis identified in two fatal cases of acute meningoencephalitis. PLoS Negl. Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0007893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Guazon M.P., Abad J. A case of infestation with Dipylidium caninum. Phil. J. Sci. 1916;11:19–31. [Google Scholar]
- Mohammed K., Tukur S.M., Watanabe M., Rani P.A.M.A., Sharma S.K.R., Fong L., Watanabe M. Molecular and serological detection of tick-borne hemopathogens among stray dogs in East Malaysia. J. Vet. Med. Res. 2017;4:1047. [Google Scholar]
- Mohd Zain S.N., Sahimin N., Pal P., Lewis J.W. Macroparasite communities in stray cat populations from urban cities in Peninsular Malaysia. Vet. Parasitol. 2013;196:469–477. doi: 10.1016/j.vetpar.2013.03.030. [DOI] [PubMed] [Google Scholar]
- Mokhtar A.S., Lim S.F., Tay S.T. Molecular detection of Anaplasma platys and Babesia gibsoni in dogs in Malaysia. Trop. Biomed. 2013;30:345–348. [PubMed] [Google Scholar]
- Mokhtar A.S., Tay S.T. Molecular detection of Rickettsia felis, Bartonella henselae, and B. clarridgeiae in fleas from domestic dogs and cats in Malaysia. Am. J. Trop. Med. Hyg. 2011;85:931–933. doi: 10.4269/ajtmh.2011.10-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momčilović S., Cantacessi C., Arsić-Arsenijević V., Otranto D., Tasić-Otašević S. Rapid diagnosis of parasitic diseases: Current scenario and future needs. Clin. Microbiol. Infect. 2019;25:290–309. doi: 10.1016/j.cmi.2018.04.028. [DOI] [PubMed] [Google Scholar]
- Moraes-Filho J., Krawczak F.S., Costa F.B., Soares J.F., Labruna M.B. Comparative evaluation of the vector competence of four South American populations of the Rhipicephalus sanguineus group for the bacterium Ehrlichia canis, the agent of canine monocytic ehrlichiosis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0139386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslim A., Fong M.Y., Mahmud R., Lau Y., Sivanandam S. Armigeres subalbatus incriminated as a vector of zoonotic Brugia pahangi filariasis in suburban Kuala Lumpur, Peninsular Malaysia. Parasit. Vectors. 2013;6:219. doi: 10.1186/1756-3305-6-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslim A., Fong M.Y., Mahmud R., Sivanandam S. Vector and reservoir host of a case of human Brugia pahangi infection in Selangor, peninsular Malaysia. Trop. Biomed. 2013;30:727–730. [PubMed] [Google Scholar]
- Narasimham M., Panda P., Mohanty I., Sahu S., Padhi S., Dash M. Dipylidium caninum infection in a child: A rare case report. Indian J. Med. Microbiol. 2013;31:82. doi: 10.4103/0255-0857.108738. [DOI] [PubMed] [Google Scholar]
- Nasirudeen A.M.A., Thong M.E.E.L. Prevalence of Bartonella henselae immunoglobulin G antibodies in Singaporean cats. Pediatr. Infect. Dis. J. 1999;18:276–278. doi: 10.1097/00006454-199903000-00014. [DOI] [PubMed] [Google Scholar]
- Nava S., Beati L., Venzal J.M., Labruna M.B., Szabó M.P.J., Petney T., Saracho-Bottero M.N., et al. Rhipicephalus sanguineus (Latreille, 1806): Neotype designation, morphological re-description of all parasitic stages and molecular characterization. Ticks Tick. Borne. Dis. 2018;9:1573–1585. doi: 10.1016/j.ttbdis.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Nazari M., Lim S.Y., Watanabe M., Sharma R.S.K., Cheng N.A.B.Y., Watanabe M. Molecular detection of Ehrlichia canis in dogs in Malaysia. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng-Nguyen D., Hii S.F., Hoang M.T.T., Nguyen V.A.T., Rees R., Stenos J., Traub R.J. Domestic dogs are mammalian reservoirs for the emerging zoonosis flea-borne spotted fever, caused by Rickettsia felis. Sci. Rep. 2020;10:4145. doi: 10.1038/s41598-020-61122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngui R., Lee S.C., Yap N.J., Tan T.K., Aidil R.M., Chua K.H., et al. Gastrointestinal parasites in rural dogs and cats in Selangor and Pahang states in Peninsular Malaysia. Acta Parasitol. 2014;59:737–744. doi: 10.2478/s11686-014-0306-3. [DOI] [PubMed] [Google Scholar]
- Nguyen V.-L., Colella V., Greco G., Fang F., Nurcahyo W., Hadi U.K., et al. Molecular detection of pathogens in ticks and fleas collected from companion dogs and cats in East and Southeast Asia. Parasit. Vectors. 2020;13:420. doi: 10.1186/s13071-020-04288-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V.L., Colella V., Iatta R., Bui K.L., Dantas-Torres F., Otranto D. Ticks and associated pathogens from dogs in northern Vietnam. Parasitol. Res. 2019;118:139–142. doi: 10.1007/s00436-018-6138-6. [DOI] [PubMed] [Google Scholar]
- Nguyen P.B., Nguyen T.T., Le T.T.H. Epidemiological survey of gastrointestinal helminth parasites of dogs in Long Xuyen city, an Giang province. AGU J. Sci. 2015;3:31–37. [Google Scholar]
- Nguyen H.M., Theppannga W., Vongphayloth K., Douangngeun B., Blacksell S.D., Robinson M.T. Screening of ectoparasites from domesticated dogs for bacterial pathogens in Vientiane, Lao PDR. Zoonoses Public Health. 2020:1–7. doi: 10.1111/zph.12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwetpathomwat A., Techangamsuwan S., Suvarnavibhaja S. A retrospective study of the clinical hematology and biochemistry of canine ehrlichiosis in an animal hospital population in Bangkok, Thailand. Comp. Clin. Path. 2006;14:217–220. doi: 10.1007/s00580-005-0587-x. [DOI] [Google Scholar]
- Noopetch P., Ponpinit T., Suankratay C. Bartonella henselae infective endocarditis with dissemination: A case report and literature review in Southeast Asia. IDCases. 2018;13 doi: 10.1016/j.idcr.2018.e00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor Azian M., Lokman Hakim S., Nagesh Khadri M.J., Yusri M.Y., Adela J.I., Noor M., et al. Leishmaniasis in peninsular Malaysia: The role of immigrant workers and the vector. Southeast Asian J. Trop. Med. Public Health. 2016;47:607–616. [Google Scholar]
- Noordin R., Shenoy R.K., Lim B.H., Ramachandran C.P. In: Parasites and their vectors: A special focus on Southeast Asia. Lim Y.A.L., Vythilingam I., editors. Springer-Verlag; Wien: 2013. Filarial worms in Southeast Asia; pp. 33–56. [DOI] [Google Scholar]
- Nuchprayoon S., Junpee A., Nithiuthai S., Chungpivat S., Suvannadabba S., Poovorawan Y. Detection of filarial parasites in domestic cats by PCR-RFLP of ITS1. Vet. Parasitol. 2006;140:366–372. doi: 10.1016/j.vetpar.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Otranto D. Diagnostic challenges and the unwritten stories of dog and cat parasites. Vet. Parasitol. 2015;212:54–61. doi: 10.1016/j.vetpar.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Otranto D. Arthropod-borne pathogens of dogs and cats: From pathways and times of transmission to disease control. Vet. Parasitol. 2018;251:68–77. doi: 10.1016/j.vetpar.2017.12.021. [DOI] [PubMed] [Google Scholar]
- Otranto D., Dantas-Torres F. The prevention of canine leishmaniasis and its impact on public health. Trends Parasitol. 2013;29:339–345. doi: 10.1016/j.pt.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Otranto D., Dantas-Torres F., Breitschwerdt E.B. Managing canine vector-borne diseases of zoonotic concern: Part two. Trends Parasitol. 2009;25:228–235. doi: 10.1016/j.pt.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Otranto D., Dantas-Torres F., Brianti E., Traversa D., Petrić D., Genchi C., Capelli G. Vector-borne helminths of dogs and humans in Europe. Parasit. Vectors. 2013;6:16. doi: 10.1186/1756-3305-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D., Dantas-Torres F., Mihalca A.D., Traub R.J., Lappin M., Baneth G. Zoonotic parasites of sheltered and stray dogs in the era of the global economic and political crisis. Trends Parasitol. 2017;33:813–825. doi: 10.1016/j.pt.2017.05.013. [DOI] [PubMed] [Google Scholar]
- Otranto D., Eberhard M.L. Zoonotic helminths affecting the human eye. Parasit. Vectors. 2011;4:41. doi: 10.1186/1756-3305-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D., Lia R.P., Buono V., Traversa D., Giangaspero A. Biology of Thelazia callipaeda (Spirurida, Thelaziidae) eyeworms in naturally infected definitive hosts. Parasitology. 2004;129:627–633. doi: 10.1017/S0031182004006018. [DOI] [PubMed] [Google Scholar]
- Otranto D., Mendoza-Roldan J.A., Dantas-Torres F. Thelazia callipaeda. Trends Parasitol. 2020;S1471–4922:30127–30136. doi: 10.1016/j.pt.2020.04.013. [DOI] [PubMed] [Google Scholar]
- Otranto D., Wall R. New strategies for the control of arthropod vectors of disease in dogs and cats. Med. Vet. Entomol. 2008;22:291–302. doi: 10.1111/j.1365-2915.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- Palmieri J.R., Masbar S., Purnomo U., Marwoto H.A., Tirtokusumo S., Darwis F. The domestic cat as a host for brugian filariasis in South Kalimantan (Borneo), Indonesia. J. Helminthol. 1985;59:277–281. doi: 10.1017/s0022149x00008099. https://doi.org/DOI: 10.1017/S0022149X00008099. [DOI] [PubMed] [Google Scholar]
- Panarese R., Iatta R., Latrofa M.S., Zatelli A., Ignjatović Ćupina A., Montarsi F., et al. Hyperendemic Dirofilaria immitis infection in a sheltered dog population: An expanding threat in the mediterranean region. Int. J. Parasitol., 2020;50:555–559. doi: 10.1016/j.ijpara.2020.04.002. [DOI] [PubMed] [Google Scholar]
- Panigrahi P.N., Mahendran K., Jena S.C., Behera P., Mahajan S., Arjun K., Dey S. Trypanosoma evansi infection in a German shepherd dog - apparent successful treatment using serial low dose of diminazene aceturate. Vet. Parasitol. Reg. Stud. Reports. 2015;1:70–74. doi: 10.1016/j.vprsr.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Parola P. Rickettsia felis: From a rare disease in the USA to a common cause of fever in sub-Saharan Africa. Clin. Microbiol. Infect. 2011;17:996–1000. doi: 10.1111/j.1469-0691.2011.03516.x. [DOI] [PubMed] [Google Scholar]
- Parola P., Miller R.S., McDaniel P., Telford S.I., Rolain J.-M., Wongsrichanalai C., Raoult D. Emerging rickettsioses of the Thai-Myanmar border. Emerg. Infect. Dis. 2003;9:592–595. doi: 10.3201/eid0905.020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola P., Sanogo O., Lerdthusnee K., Zeaiter Z., Chauvancy G., Gonzalez J., et al. Identification of Rickettsia spp. and Bartonella spp. in fleas from the Thai-Myanmar border. Ann. N. Y. Acad. Sci. 2003;990:173–181. doi: 10.1111/j.1749-6632.2003.tb07359.x. [DOI] [PubMed] [Google Scholar]
- Petney T.N., Saijuntha W., Boulanger N., Chitimia-Dobler L., Pfeffer M., Eamudomkarn C., et al. Ticks (Argasidae, Ixodidae) and tick-borne diseases of continental Southeast Asia. Zootaxa. 2019;4558:1–89. doi: 10.11646/zootaxa.4558.1.1. [DOI] [PubMed] [Google Scholar]
- Phoosangwalthong P., Hii S.F., Kamyingkird K., Kengradomkij C., Pinyopanuwat N., Chimnoi W., et al. Cats as potential mammalian reservoirs for Rickettsia sp. genotype RF2125 in Bangkok, Thailand. Vet. Parasitol. Reg. Stud. Reports. 2018;13:188–192. doi: 10.1016/j.vprsr.2018.07.001. https://doi.org/10.1016/j.vprsr.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Pinyoowong D., Jittapalapong S., Suksawat F., Stich R.W., Thamchaipenet A. Molecular characterization of Thai Ehrlichia canis and Anaplasma platys strains detected in dogs. Infect. Genet. Evol. 2008;8:433–438. doi: 10.1016/j.meegid.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Piratae S., Pimpjong K., Vaisusuk K., Chatan W. Molecular detection of Ehrlichia canis, Hepatozoon canis and Babesia canis vogeli in stray dogs in Mahasarakham province, Thailand. Ann. Parasitol. 2015;61:183–187. doi: 10.17420/ap6103.05. [DOI] [PubMed] [Google Scholar]
- Powar R.M., Shegokar V.R., Joshi P.P., Dani V.S., Tankhiwale N.S., Truc P., et al. A rare case of human trypanosomiasis caused by Trypanosoma evansi. Indian J. Med. Microbiol. 2006;24:72–74. doi: 10.4103/0255-0857.19904. [DOI] [PubMed] [Google Scholar]
- Pradatsundarasar A. Dirofilaria infection in man: Report of a case. J. Med. Assoc. Thai. 1985;38:378–379. [Google Scholar]
- Prakash B.K., Low V.L., Tan T.K., Vinnie-Siow W.Y., Lim Y.A.-L., Morvarid A.R., et al. Detection of Hepatozoon canis in the brown dog tick and domestic dogs in Peninsular Malaysia. J. Med. Entomol. 2018;55:1–3. doi: 10.1093/jme/tjy081. [DOI] [PubMed] [Google Scholar]
- Prakash B.K., Low V.L., Vinnie-Siow W.Y., Tan T.K., Lim Y.A.-L., Morvarid A.R., et al. Detection of Babesia spp. in dogs and their ticks from peninsular Malaysia: Emphasis on Babesia gibsoni and Babesia vogeli infections in Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) J. Med. Entomol. 2018;55:1337–1340. doi: 10.1093/jme/tjy072. [DOI] [PubMed] [Google Scholar]
- Rajamanickam C., Wiesenhutter E., Zin F.M., Hamid J. The incidence of canine haematozoa in peninsular Malaysia. Vet. Parasitol. 1985;17:151–157. doi: 10.1016/0304-4017(85)90101-3. https://doi.org/10.1016/0304-4017(85)90101-3. [DOI] [PubMed] [Google Scholar]
- Rashid I., Firyal S., Tariq A., Muhammad G., Saqib M., Zafar M.S. Use of Cymelarsan® and manganese chloride for treatment of the canine trypanosomiasis (surra): A research report. Adv. Anim. Vet. Sci. 2014;2:104–105. [Google Scholar]
- Rattanavong S., Fournier P.E., Chu V., Frichitthavong K., Kesone P., Mayxay M., et al. Bartonella henselae endocarditis in Laos – ‘The unsought will go undetected’. PLoS Negl. Trop. Dis. 2014;8:e3385. doi: 10.1371/journal.pntd.0003385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rjeibi M.R., Ben Hamida T., Dalgatova Z., Mahjoub T., Rejeb A., Dridi W., Gharbi M. First report of surra (Trypanosoma evansi infection) in a Tunisian dog. Parasite. 2015;22:3. doi: 10.1051/parasite/2015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohela M., Ibrahim J., Hui T., Mak J.W., Ithoi I., Amir A. Dirofilaria causing eye infection in a patient from Malaysia. Southeast Asian J. Trop. Med. Public Health. 2009;40:914–918. [PubMed] [Google Scholar]
- Rojekittikhun W., Chaisiri K., Mahittikorn A., Pubampen S., Sa-Nguankiat S., Kusolsuk T., et al. Gastrointestinal parasites of dogs and cats in a refuge in Nakhon Nayok, Thailand. Southeast Asian J Trop Med Public Health. 2014;45:31–39. [PubMed] [Google Scholar]
- Salakij C., Lertwatcharasarakul P., Salakij J., Nunklang K., Rattanakunuprakarn J. Molecular characterization of Anaplasma platys in a domestic cat from Thailand. Comp. Clin. Path. 2012;21:345–348. doi: 10.1007/s00580-011-1378-1. [DOI] [Google Scholar]
- Sapp S.G.H., Bradbury R.S. The forgotten exotic tapeworms: A review of uncommon zoonotic cyclophyllidea. Parasitology. 2020;147:533–558. doi: 10.1017/S003118202000013X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satjawongvanit H., Phumee A., Tiawsirisup S., Sungpradit S., Brownell N., Siriyasatien P., Preativatanyou K. Molecular analysis of canine filaria and its Wolbachia endosymbionts in domestic dogs collected from two animal university hospitals in Bangkok metropolitan region, Thailand. Pathogens. 2019;8:114. doi: 10.3390/pathogens8030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher J.F. Morphology of the microfilaria of Brugia pahangi and of the larval stages in the mosquito. J. Parasitol. 1962;48:679. doi: 10.2307/3275257. [DOI] [PubMed] [Google Scholar]
- Scholz T., Uhlířová M., Ditrich O. Helminth parasites of cats from the Vientiane province, Laos, as indicators of the occurrence of causative agents of human parasitoses. Parasite. 2003;10:343–350. doi: 10.1051/parasite/2003104343. [DOI] [PubMed] [Google Scholar]
- Senevtratna P., Weerasinghe N., Ariyadasa S. Transmission of Haemobartonella canis by the dog tick, Rhipiccphalus sanguineus. Res. Vet. Sci. 1973;14:112–114. https://doi.org/10.1016/S0034-5288(18)33950-X. [PubMed] [Google Scholar]
- Shah G., Shri R., Panchal V., Sharma N., Singh B., Mann A.S. Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (Lemon grass) J. Adv. Pharm. Technol. Res. 2011;2:3–8. doi: 10.4103/2231-4040.79796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard D.S., Undurraga E.A., Halasa Y.A. Economic and disease burden of dengue in Southeast Asia. PLoS Negl. Trop. Dis. 2013;7:e2055. doi: 10.1371/journal.pntd.0002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simking P., Wongnakphet S., Stich R.W., Jittapalapong S. Detection of Babesia vogeli in stray cats of metropolitan Bangkok, Thailand. Vet. Parasitol. 2010;173:70–75. doi: 10.1016/j.vetpar.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Simón F., Siles-Lucas M., Morchón R., González-Miguel J., Mellado I., Carretón E., Montoya-Alonso J.A. Human and animal dirofilariasis: The emergence of a zoonotic mosaic. Clin. Microbiol. Rev. 2012;25:507–544. doi: 10.1128/CMR.00012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer G.A., Loya F.P., Lapsley W.D., Tobar B.Z., Carlos S., Carlos R.S., et al. Detection of Bartonella infection in pet dogs from Manila, the Philippines. Acta Trop. 2020;205:105277. doi: 10.1016/j.actatropica.2019.105277. [DOI] [PubMed] [Google Scholar]
- Soto F., Walker R., Sepulveda M., Bittencourt P., Acosta-Jamett G., Müller A. Occurrence of canine hemotropic mycoplasmas in domestic dogs from urban and rural areas of the Valdivia Province, southern Chile. Comp. Immunol. Microbiol. Infect. Dis. 2017;50:70–77. doi: 10.1016/j.cimid.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Srisanyong W., Takhampunya R., Boonmars T., Kerdsin A., Suksawat F. Prevalence of Bartonella henselae, Bartonella clarridgeiae, and Bartonella vinsonii subsp. berkhoffii in pet cats from four provincial communities in Thailand. Thai J Vet Med. 2016;46:663–670. [Google Scholar]
- Sthitmatee N., Jinawan W., Jaisan N., Tangjitjaroen W., Chailangkarn S., Sodarat C., et al. Genetic and immunological evidences of Borrelia burgdorferi in dog in Thailand. Southeast Asian J. Trop. Med. Public Health. 2016;47:71–77. [PubMed] [Google Scholar]
- Sudomo M., Chayabejara S., Duong S., Hernandez L., Wu W.P., Bergquist R. Elimination of lymphatic filariasis in Southeast Asia. Adv. Parasitol. 2010;72:205–233. doi: 10.1016/S0065-308X(10)72008-X. [DOI] [PubMed] [Google Scholar]
- Sukhumavasi W., Bellosa M.L., Lucio-Forster A., Liotta J.L., Lee A.C.Y., Pornmingmas P., et al. Serological survey of Toxoplasma gondii, Dirofilaria immitis, Feline immunodeficiency virus (FIV) and Feline Leukemia virus (FeLV) infections in pet cats in bangkok and vicinities, Thailand. Vet. Parasitol. 2012;188:25–30. doi: 10.1016/j.vetpar.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Sukpanichnant S., Leenuttapong V., Dejsomritrutai W., Thakerngpol K., Wanachiwanawin W., Kachintorn U., Nitiyanant W. Pulmonary dirofilariasis in a patient with multisystem Langerhans cell histiocytosis-the first reported case in Thailand. J. Med. Assoc. Thai. 1998;81:722–727. [PubMed] [Google Scholar]
- Suksawat J., Xuejie Y., Hancock S.I., Hegarty B.C., Nilkumhang P., Breitschwerdt E.B. Serologic and molecular evidence of coinfection with multiple vector-borne pathogens in dogs from Thailand. J. Vet. Intern. Med. 2001;15:453–462. doi: 10.1111/j.1939-1676.2001.tb01574.x. [DOI] [PubMed] [Google Scholar]
- Sukudom P., Phumee A., Siriyasatien P. First report on subconjunctival dirofilariasis in Thailand caused by a Dirofilaria sp. closely related to D. hongkongensis . Acad. J. Sci. Res. 2018;6:114–116. [Google Scholar]
- Tan C.L., Fhun L.C., Tai E.L.M., Abdul Gani N.H., Muhammed J., Tuan Jaafar T.N., et al. Clinical profile and visual outcome of ocular bartonellosis in Malaysia. J. Trop. Med. 2017;2017:7946123. doi: 10.1155/2017/7946123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L.H., Fong M.Y., Mahmud R., Muslim A., Lau Y.L., Kamarulzaman A. Zoonotic Brugia pahangi filariasis in a suburbia of Kuala Lumpur city, Malaysia. Parasitol. Int. 2011;60:111–113. doi: 10.1016/j.parint.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Tanskul P., Stark H., Inlao I. A checklist of ticks of Thailand (Acari: Metastigmata: Ixodoidea) J. Med. Entomol. 1983;20:330–341. doi: 10.1093/JMEDENT/20.3.330. [DOI] [PubMed] [Google Scholar]
- Tawatsin A., Thavara U., Wongsinkongman P., Bansidhi J., Boonruad T., Chavalittumrong P., et al. Repellency of essential oils extracted from plants in Thailand against four mosquito vectors (Diptera: Culicidae) and oviposition deterrent effects against Aedes aegypti (Diptera: Culicidae) Southeast Asian J. Trop. Med. Public Health. 2006;37:915–931. [PubMed] [Google Scholar]
- Taylor A.E.R. Studies on the microfilariae of Loa loa, Wuchereria bancrofti, Brugia malayi, Dirofilaria immitis, D. repens and D. aethiops. J. Helminthol. 1960;34:13–26. doi: 10.1017/S0022149X00020290. [DOI] [PubMed] [Google Scholar]
- Thanchomnang T., Intapan P.M., Chungpivat S., Lulitanond V., Maleewong W. Differential detection of Brugia malayi and Brugia pahangi by real-time fluorescence resonance energy transfer PCR and its evaluation for diagnosis of B. pahangi-infected dogs. Parasitol. Res. 2010;106:621–625. doi: 10.1007/s00436-009-1706-4. [DOI] [PubMed] [Google Scholar]
- Thanchomnang T., Intapan P.M., Tantrawatpan C., Lulitanond V., Chungpivat S., Taweethavonsawat P., et al. Rapid detection and identification of Wuchereria bancrofti, Brugia malayi, B. pahangi, and Dirofilaria immitis in mosquito vectors and blood samples by high resolution melting real-time PCR. Korean J. Parasitol. 2013;51:645–650. doi: 10.3347/kjp.2013.51.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The World Bank World population prospects: 2019 revision. 2019. https://data.worldbank.org/indicator/SP.POP.TOTL
- Theis J.H., Tran S.T., Carlos M.P., Carlos E.T., Carlos S.M.E. Incidence of Dirofilaria immitis (Leidy) in client owned dogs from the greater metropolitan Manila area of Luzon island in the Philippines. Open Vet. Sci. J. 2008;2:50–54. doi: 10.2174/1874318808002010050. [DOI] [Google Scholar]
- Tiawsirisup S., Kaewthamasorn M. The potential for Aedes albopictus (Skuse) (Diptera: Culicidae) to be a competent vector for canine heartworm, Dirofilaria immitis (Leidy) Southeast Asian J. Trop. Med. Public Health. 2007;38:208–214. [Google Scholar]
- Tiawsirisup S., Nithiuthai S. Vector competence of Aedes aegypti (L.) and Culex quinquefasciatus (Say) for Dirofilaria immitis (Leidy) Southeast Asian J. Trop. Med. Public Health. 2006;37:110–114. [PubMed] [Google Scholar]
- Tiawsirisup S., Nithiuthai S., Kaewthamasorn M. Repellent and adulticide efficacy of a combination containing 10% imidacloprid and 50% permethrin against Aedes aegypti mosquitoes on dogs. Parasitol. Res. 2007;101:527–531. doi: 10.1007/s00436-007-0508-9. [DOI] [PubMed] [Google Scholar]
- Tinkruejeen G., Meesaimongkon P., Tangtrongsup S., Thitaram N., Srifawattana N., Beugnet F., Tiwananthagorn S. Comparative efficacy of afoxolaner and ivermectin in dogs naturally infested with Rhipicephalus sanguineus sensu lato: A clinical field study conducted in Thailand. Vet. Parasitol. Reg. Stud. Reports. 2019;18:100340. doi: 10.1016/j.vprsr.2019.100340. [DOI] [PubMed] [Google Scholar]
- Tisgratog R., Sanguanpong U., Grieco J.P., Ngoen-Kluan R., Chareonviriyaphap T. Plants traditionally used as mosquito repellents and the implication for their use in vector control. Acta Trop. 2016;157:136–144. doi: 10.1016/j.actatropica.2016.01.024. [DOI] [PubMed] [Google Scholar]
- To K.K.W., Wong S.S.Y., Poon R.W.S., Trendell-Smith N.J., Ngan A.H.Y., Lam J.W.K., et al. A novel Dirofilaria species causing human and canine infections in Hong Kong. J. Clin. Microbiol. 2012;50:3534–3541. doi: 10.1128/JCM.01590-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukur S.M., Mohammed K., Watanabe M., Rani P.A.M.A., Watanabe M. Coxiella burnetii detection in stray dogs in Klang Valley, Malaysia. J. Adv. Microbiol., 2019;14:1–7. [Google Scholar]
- Van De N., Le T.H., Chai J.Y. Dirofilaria repens in Vietnam: Detection of 10 eye and subcutaneous tissue infection cases identified by morphology and molecular methods. Korean J. Parasitol. 2012;50:137–141. doi: 10.3347/kjp.2012.50.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vinh Chau N., Buu Chau L., Desquesnes M., Herder S., Phu Huong Lan N., Campbell J.I., et al. A clinical and epidemiological investigation of the first reported human infection with the zoonotic parasite Trypanosoma evansi in Southeast Asia. Clin. Infect. Dis. 2016;62:1002–1008. doi: 10.1093/cid/ciw052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varagnol M., Parola P., Jouan R., Beaucournu J.C., Rolain J.M., Raoult D. First detection of Rickettsia felis and Bartonella clarridgeiae in fleas from Laos. Clin. Microbiol. Infect. 2009;15:334–335. doi: 10.1111/j.1469-0691.2008.02272.x. [DOI] [PubMed] [Google Scholar]
- Verloo D., Holland W., My L.N., Thanh N.G., Tam P.T., Goddeeris B., Vercruysse J., Büscher P. Comparison of serological tests for Trypanosoma evansi natural infections in water buffaloes from north Vietnam. Vet. Parasitol. 2000;92:87–96. doi: 10.1016/s0304-4017(00)00284-3. [DOI] [PubMed] [Google Scholar]
- Viriyavejakul P., Krudsood S., Monkhonmu S., Punsawad C., Riganti M., Radomyos P. Thelazia callipaeda: A human case report. Southeast Asian J. Trop. Med. Public Health. 2012;43:851–856. [PubMed] [Google Scholar]
- Vu S.N., Nguyen T.Y., Tran V.P., Truong U.N., Le Q.M., Le V.L., et al. Elimination of dengue by community programs using Mesocyclops (Copepoda) against Aedes aegypti in central Vietnam. Am. J. Trop. Med. Hyg. 2005;72:67–73. [PubMed] [Google Scholar]
- Watanabe M., Nakao R., Amin-Babjee S.M.S., Maizatul A.M.A., Youn J.H.J., Qiu Y., et al. Molecular screening for Rickettsia, Anaplasmataceae and Coxiella burnetii in Rhipicephalus sanguineus ticks from Malaysia. Trop. Biomed. 2015;32:390–398. [PubMed] [Google Scholar]
- Watt G., Pachirat O., Baggett H.C., Maloney S.A., Lulitanond V., Raoult D., et al. Infective endocarditis in northeastern Thailand. Emerg. Infect. Dis. 2014;20:473–476. doi: 10.3201/eid2003.131059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willi B., Boretti F.S., Tasker S., Meli M.L., Wengi N., Reusch C.E., et al. From haemobartonella to hemoplasma: Molecular methods provide new insights. Vet. Microbiol. 2007;125:197–209. doi: 10.1016/j.vetmic.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Wolf S., Reeves W. Rickettsia felis (Rickettsiales: Rickettsiaceae) discovered in cat fleas (Siphonaptera: Pulicidae) in the Philippines. J. Entomol. Sci. 2012;47:95–96. [Google Scholar]
- Wongkamchai S., Nochote H., Foongladda S., Dekumyoy P., Thammapalo S., Boitano J.J., Choochote W. A high resolution melting real time PCR for mapping of filaria infection in domestic cats living in brugian filariosis-endemic areas. Vet. Parasitol. 2014;201:120–127. doi: 10.1016/j.vetpar.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Woods J.E., Brewer M.M., Hawley J.R., Wisnewski N., Lappin M.R. Evaluation of experimental transmission of Candidatus Mycoplasma haemominutum and Mycoplasma haemofelis by Ctenocephalides felis to cats. Am. J. Vet. Res. 2005;66:1008–1012. doi: 10.2460/ajvr.2005.66.1008. [DOI] [PubMed] [Google Scholar]
- World Health Organization Lymphatic filariasis: Progress report 2000–2009 and strategic plan 2010–2020. 2010. https://www.who.int/lymphatic_filariasis/resources/9789241500722/en/
- World Health Organization World malaria report 2019. 2019. https://www.who.int/publications/i/item/9789241565721
- World Health Organization Lymphatic filariasis. 2020. https://www.who.int/gho/neglected_diseases/lymphatic_filariasis/en/
- Ybañez A.P., Perez Z.O., Gabotero S.R., Yandug R.T., Kotaro M., Inokuma H. First molecular detection of Ehrlichia canis and Anaplasma platys in ticks from dogs in Cebu, Philippines. Ticks Tick. Borne. Dis. 2012;3:288–293. doi: 10.1016/j.ttbdis.2012.10.032. [DOI] [PubMed] [Google Scholar]
- Ybañez A.P., Ybañez R.H.D., Talle M.F.G., Liu M., Moumouni P.F.A., Xuan X. First report on Babesia vogeli infection in dogs in the Philippines. Parasitol. Int. 2017;66:813–815. doi: 10.1016/j.parint.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Ybañez A.P., Ybañez R.H.D., Yokoyama N., Inokuma H. Multiple infections of Anaplasma platys variants in Philippine dogs. Vet. World. 2016;9:1456–1460. doi: 10.14202/vetworld.2016.1456-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz E., Fritzenwanker M., Pantchev N., Lendner M., Wongkamchai S., Otranto D., et al. The mitochondrial genomes of the zoonotic canine filarial parasites Dirofilaria (Nochtiella) repens and Candidatus Dirofilaria (Nochtiella) honkongensis provide evidence for presence of cryptic species. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0005028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz E., Wongkamchai S., Ramünke S., Koutsovoulos G.D., Blaxter M.L., Poppert S., et al. High genetic diversity in the Dirofilaria repens species complex revealed by mitochondrial genomes of feline microfilaria samples from Narathiwat, Thailand. Transbound. Emerg. Dis. 2019;66:389–399. doi: 10.1111/tbed.13033. [DOI] [PubMed] [Google Scholar]
- Yospaiboon Y., Sithithavorn P., Maleewong V., Ukosanakarn U., Bhaibulaya M. Ocular thelaziasis in Thailand: A case report. J. Med. Assoc. Thai. 1989;72:469–473. [PubMed] [Google Scholar]
- Zielke E., Hinz E.A., Sucharit S. Lymphatic filariasis in Thailand: A review on distribution and transmission. Tropenmed. Parasitol. 1993;15:141–148. [Google Scholar]