Fig. 4: A vision for probabilistic risk assessment (ProbRA) of substances.
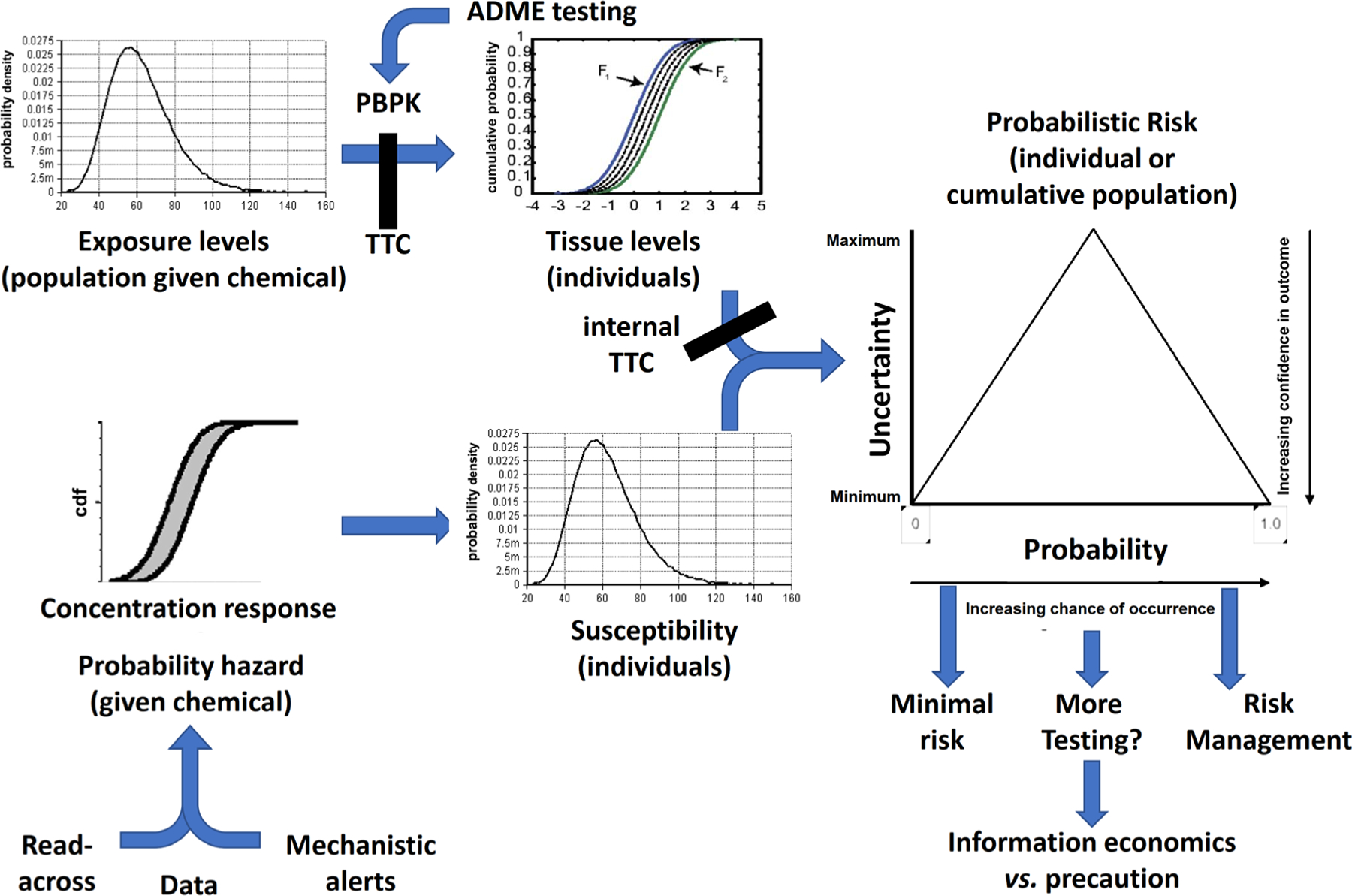
ProbRA is fueled by probability of exposure and probability of hazard and susceptibility. Exposure is first characterized by a population distribution (cumulative from the individuals’ exposure distributions). Where they do not exceed applicable thresholds of toxicological concern (TTC), the assessment might be abrogated on the ground of negligible exposure. Probabilistic physiology-based pharmacokinetic (or toxicokinetic, respectively) modeling (PBPK) translates these into resulting tissue concentrations. This can be refined by adsorption, metabolism, distribution & excretion (ADME) measurements or estimates. Internal TTC again might allow to abrogate the assessment in case of irrelevant tissue level concentrations. The second line of evidence is establishing the probability of hazard. This can be based on mechanistic data, mechanistic tests, and read-across to similar chemicals and any combination thereof. This probability is ideally combined with a distribution of susceptibility of different individuals. Together, tissue level concentrations and hazard probabilities give a probabilistic risk for an individual and cumulatively for the population. Low risk can lead to deprioritization depending on the use scenario, while high risk should lead to classification and risk management measures as appropriate. Intermediate probabilities of risk, i.e., high uncertainties, should be considered for additional testing, ideally considering the economics of possible information gain, or precautionary risk management.
