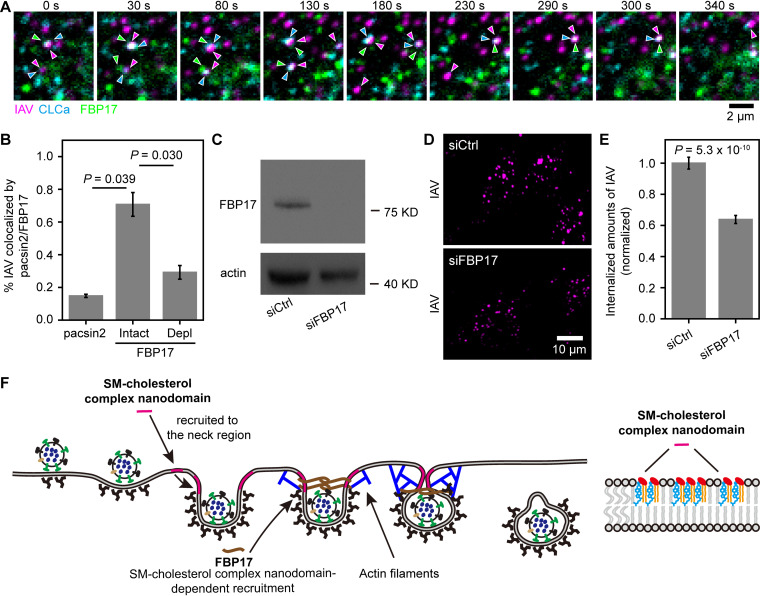
FIG 7.
SM-sequestered cholesterol is required for the recruitment of FBP17 to the IAV-CCS complex during IAV entry. (A) A typical tricolor confocal image sequence showing that FBP17 became colocalized with a CCS bound by an IAV virion. Images were obtained every 2 s. Three typical events are shown in this image sequence. (B) SM-sequestered cholesterol is required for the recruitment of FBP17 in IAV cell entry, whereas pacsin2 is not involved in IAV cell entry. pacsin2, 65 IAVs in 20 cells; FBP17-Intact, 102 IAVs in 35 cells; FBP17-Depl, 78 IAVs in 42 cells. (C) Western blot pattern showing that transfection of an siRNA targeting FBP17 (siFBP17) reduced the expression of FBP17 by over 90%. siCtrl, control siRNA. (D) Representative confocal images of internalized IAV in cells without (among 24 cells) or after (among 30 images) FBP17 depletion showing that FBP17 is involved in IAV internalization. (E) Depletion of FBP17 reduced IAV internalization by ∼40%. (F) Schematic model depicting the role of the SM-cholesterol complex nanodomain in CME of IAV. The SM-cholesterol complex nanodomain is recruited to the IAV-containing CCS. Due to the high bending rigidity of the SM-cholesterol nanodomain, when it is recruited to the neck region of the CCS, it could press the IAV-bound region of the CCS inward. The SM-cholesterol complex nanodomain is also essential for the recruitment of FBP17, a membrane-bending protein, which activates actin assembly, inducing the constriction of the IAV-containing CCP.