To the Editor:
The role of trunk inclination on respiratory function has been explored in patients with “typical” acute respiratory distress syndrome (ARDS) (1–3). Data regarding patients with coronavirus disease (COVID-19)–associated ARDS (C-ARDS) are currently lacking.
The aim of our study was to assess the effects of changes in trunk inclination on lung mechanics and gas exchange in mechanically ventilated patients with C-ARDS.
Methods
This single-center physiological crossover study (ethical committee approval #70-11022021) was conducted on adult patients admitted to our COVID-ICU between March 3 and May 4, 2021. Diagnosis of C-ARDS, deep sedation, paralysis, and volume-controlled mechanical ventilation were the inclusion criteria. Contraindications to mobilization (e.g., intracranial hypertension, spinal cord injury, tracheal lesions) and pregnancy constituted exclusion criteria. Patients were enrolled according to study personnel availability. A 5-F esophageal balloon (CooperSurgical) was inserted. The balloon was inflated with 1 ml of air, and the correct position/function was verified before each measurement (4). Mechanical ventilation parameters, kept constant throughout the study, were set by the attending physician. Usually, positive end-expiratory pressure (PEEP) is set according to the best respiratory system compliance (CRS) assessed with a recruitment maneuver followed by a decremental PEEP trial. Of note, trunk inclination during PEEP selection is not standardized.
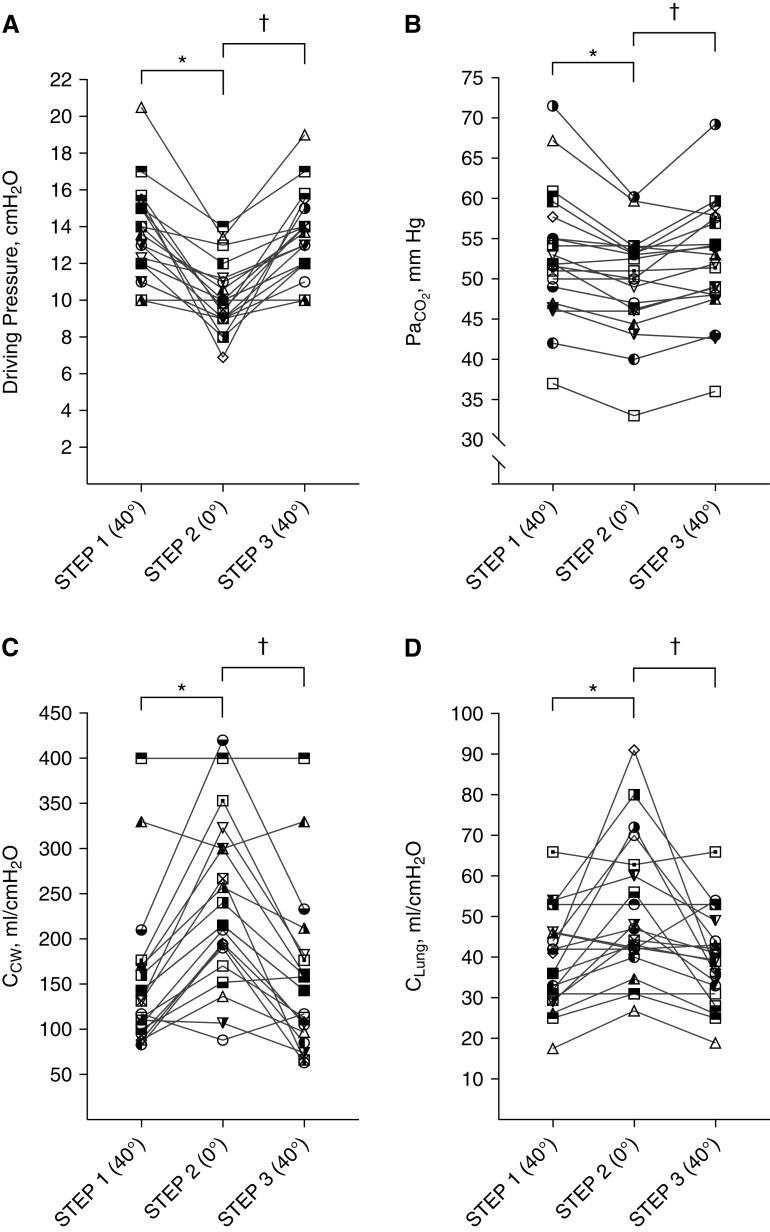
Patients underwent three 15-minute steps in which trunk inclination was changed from 40° (semirecumbent, baseline) to 0° (supine-flat), and back to 40° during the last step. At the end of each step, partitioned respiratory mechanics, arterial/central venous blood gas analysis, and basic hemodynamics were recorded. Ventilatory ratio was calculated.
Statistical analysis
Continuous variables are expressed as median (interquartile range). One-way ANOVA for repeated measures or the Friedman test was applied, as appropriate. Bonferroni and Dunn’s post hoc comparisons were used, respectively. A P < 0.05 was considered statistically significant (GraphPad Software).
Results
Twenty patients were enrolled (11 male; 67 [59–70] years; body mass index, 30 [28–35] kg/m2; Simplified Acute Physiology Score-II, 36 [32–45]). ARDS was mild in 1, moderate in 9, and severe in 10 patients. Patients were studied 2.5 (2.0–4.5) days after intubation. Vt was 5.9 (5.7–6.3) ml/kg of predicted body weight, and PEEP was 14 (12–14) cm H2O.
After changing trunk inclination from 40° to 0°, driving pressure decreased from 13 (12–15) to 10 (9–11) cm H2O (P < 0.0001) and CRS increased from 29 (24–35) to 38 (33–48) ml/cm H2O (P < 0.0001). Compared with the values obtained at baseline (semirecumbent), the supine-flat position was associated with increased chest wall compliance (CCW) (131 [101–170] vs. 215 [175–300] ml/cm H2O; P < 0.01) and increased lung compliance (CLung) (38 [30–46] vs. 46 [40–62] ml/cm H2O; P < 0.01).
A significant reduction in both PaCO2 (52 [47–57] vs. 50 [46–54] mm Hg; P < 0.001) and ventilatory ratio (1.81 [1.47–2.02] vs. 1.68 [1.43–1.96]; P < 0.001) was recorded when patients were placed supine-flat. Moreover, a positive correlation (r = 0.66; P = 0.002) between the drop of driving pressure and the reduction of PaCO2 was observed. Oxygenation was not significantly affected by changes in trunk inclination. Changes in respiratory mechanics and PaCO2 were rapidly reversed once patients were repositioned in the semirecumbent position (Table 1 and Figure 1).
Table 1.
Effect of Trunk Inclination on Ventilatory Parameters, Gas Exchange, and Hemodynamics
| First Step (40°) | Second Step (0°) | Third Step (40°) | P Value | |
|---|---|---|---|---|
| Ventilatory parameters | ||||
| Peak inspiratory pressure, cm H2O | 32 (29–36) | 28 (26–34)* | 32 (29–36)† | <0.0001 |
| Mean airway pressure, cm H2O | 18 (16–19) | 17 (16–19)* | 18 (16–19)† | <0.01 |
| Plateau pressure, cm H2O | 27 (25–28) | 24 (21–25)* | 27 (26–28)† | <0.0001 |
| End-expiratory airway pressure, cm H2O | 14 (12–14) | 14 (12–14) | 14 (12–14) | 0.47 |
| Driving pressure, cm H2O | 13 (12–15) | 10 (9–11)* | 13 (12–15)† | <0.0001 |
| End-inspiratory esophageal pressure, cm H2O | 11 (9–16) | 14 (13–17)* | 11 (10–16)† | <0.001 |
| End-expiratory esophageal pressure, cm H2O | 8 (6–14) | 12 (11–16)* | 9 (6–13)† | <0.0001 |
| End-inspiratory transpulmonary pressure, PLes, cm H2O | 15 (13–18) | 9 (7–10)* | 14 (12–17)† | <0.0001 |
| End-inspiratory transpulmonary pressure, PLer, cm H2O | 20 (19–23) | 19 (17–22)* | 20 (18–22) | 0.027 |
| Driving transpulmonary pressure, cm H2O | 10 (8–12) | 8 (6–10)* | 10 (8–12)† | <0.01 |
| CRS, ml/cm H2O | 29 (24–35) | 38 (33–48)* | 29 (24–35)† | <0.0001 |
| CCW, ml/cm H2O | 131 (101–170) | 215 (175–300)* | 143 (99–181)† | <0.001 |
| CLung, ml/cm H2O | 38 (30–46) | 46 (40–62)* | 39 (31–48)† | <0.01 |
| Gas exchange and ABG parameters | ||||
| PaO2/FiO2 | 145 (115–189) | 140 (102–175) | 144 (109–181) | 0.74 |
| SaO2, % | 96 (95–97) | 96 (94–98) | 96 (95–97) | 0.94 |
| PaCO2, mm Hg | 52 (47–57) | 50 (46–54)* | 52 (48–57)† | <0.001 |
| pH | 7.39 (7.35–7.42) | 7.38 (7.36–7.43)* | 7.39 (7.34–7.42)† | <0.01 |
| Lactate, mmol/L | 1.14 (0.9–1.4) | 1.11 (0.9–1.4) | 1.14 (0.9–1.4) | 0.82 |
| Shunt, % (n = 19) | 33 (23–42) | 33 (26–42) | 34 (26–40) | 0.70 |
| Ventilatory ratio | 1.81 (1.47–2.02) | 1.68 (1.43–1.96)* | 1.77 (1.39–2.01)† | <0.001 |
| Hemodynamics | ||||
| HR, n/min | 75 (58–85) | 74 (54–89) | 77 (56–88) | 0.25 |
| MAP, mm Hg | 78 (71–88) | 84 (73–92) | 81 (73–89) | 0.23 |
| CVP, mm Hg | 8 (6–10) | 10 (8–12) | 8 (6–10) | 0.036 |
Definition of abbreviations: ABG = arterial blood gas; CCW = chest wall compliance; CLung = lung compliance; CRS = compliance of the respiratory system; CVP = central venous pressure; HR = heart rate; MAP = mean arterial pressure; PLer = end-inspiratory transpulmonary pressure calculated from elastance ratio (10); PLes = end-inspiratory transpulmonary pressure calculated from esophageal pressure.
Data are expressed as median (interquartile range).
P < 0.05 second step (0°) versus first step (40°).
P < 0.05 third step (40°) versus second step (0°).
Figure 1.
Driving pressure, PaCO2, chest wall compliance, and lung compliance. (A) Driving pressure, (B) PaCO2, (C) chest wall compliance, and (D) lung compliance have been reported as individual values. A combination of symbol and color was assigned to each patient and was kept constant in the four graphs to allow their identification. *P < 0.05 second step (0°) versus first step (40°); †P < 0.05 third step (40°) versus second step (0°). CCW = chest wall compliance; CLung = lung compliance.
Discussion
The change in trunk inclination from semirecumbent to supine-flat in patients with C-ARDS: 1) increased CRS owing to both an increase in CCW and CLung; 2) improved CO2 clearance; and 3) had no considerable effect on oxygenation.
These findings have several implications. First, it is of interest to understand the mechanisms leading to such a remarkable, quick, and reversible improvement in the mechanical characteristics of the respiratory system. Compliance improvements in patients with ARDS are frequently attributed to the recruitment of previously collapsed alveoli, and therefore to an increase in end-expiratory lung volume (EELV). Another possible mechanism is a certain degree of lung derecruitment accompanied by intratidal recruitment (5). Finally, the reduction in overdistension of previously overstretched lung regions could play a role (i.e., a reduction in the aeration of ventilated alveoli) (6).
Our results are not sufficient to clearly identify the underlying mechanisms, as we did not assess EELV and regional ventilation distribution. However, a major role of alveolar recruitment is unlikely in the present context, given the rapidity of the observed improvement and its reversibility once placed back in the semirecumbent position. Moreover, although intratidal recruitment might play a role, we think that the major pathophysiological mechanism likely explaining our findings is a reduced overdistention of some lung regions. In other words, it is conceivable that placing patients in the supine position caused a cephalad displacement of the diaphragm, resulting in a reduction in EELV and alveolar overdistension. This hypothesis is upheld by both the available literature, demonstrating that the supine-flat position is associated with a reduction in EELV (1–3, 7) and by the significant reduction in PaCO2 in this position.
Our results regarding changes in respiratory mechanics are in line with previous studies performed on patients with typical ARDS (1–3). Put together, these studies convey a clear clinical and methodological message: trunk inclination should be measured and reported when assessing respiratory mechanics. From a clinical perspective, to monitor patients’ respiratory mechanics, it appears to be of the utmost importance to standardize trunk inclination when respiratory mechanics are assessed. In addition, although there is likely no correct angle, we think that trunk inclination should always be stated in the methods to improve the reliability and reproducibility of clinical studies dealing with respiratory mechanics. Another important clinical implication of our study is that a simple intervention, such as placing the patient supine-flat, markedly reduces driving pressure and lung stress (transpulmonary pressure) (8). Moreover, the improved CO2 clearance could potentially allow reduction of the respiratory rate, further lowering the mechanical power delivered to the lungs and thus the risk of ventilator-induced lung injury (9).
As the study steps were relatively short, we can draw no conclusions on the long-term effects on gas exchange and ventilator-induced lung injury. Other limitations of our study are the lack of gastric pressure measurement and the high and relatively homogenous PEEP levels.
Conclusions
The change in body position from semirecumbent to supine-flat improved respiratory mechanics and CO2 clearance and did not worsen oxygenation in C-ARDS. Given the remarkable effect of trunk inclination on respiratory mechanics, we think that reporting the angle of trunk inclination is of extreme importance to obtain a reliable assessment and monitoring of the respiratory mechanics in mechanically ventilated patients.
Acknowledgments
Acknowledgments
The authors thank Gianpaola Monti, M.D.; Paola Previtali, M.D.; Jacopo Colombo, M.D.; Fernando Arnaiz Guerrero, M.D.; Manuela Paradiso, M.D.; Nicola Suardi, M.D.; Chiara Borromeo, M.D.; and all the physicians and nurses of the Rossini and other COVID-19 intensive care units of Niguarda Hospital.
Footnotes
Author Contributions: F.M., S.S., R.F., and T.L. conceived the study. F.M., S.S., and T.L. performed data analysis and prepared the first draft of the manuscript. All authors were responsible for data acquisition and interpretation, reviewed the manuscript, and approved the final submitted version.
Originally Published in Press as DOI: 10.1164/rccm.202110-2360LE on January 4, 2022
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Mezidi M, Guérin C. Effect of body position and inclination in supine and prone position on respiratory mechanics in acute respiratory distress syndrome. Intensive Care Med . 2019;45:292–294. doi: 10.1007/s00134-018-5493-1. [DOI] [PubMed] [Google Scholar]
- 2. Dellamonica J, Lerolle N, Sargentini C, Hubert S, Beduneau G, Di Marco F, et al. Effect of different seated positions on lung volume and oxygenation in acute respiratory distress syndrome. Intensive Care Med . 2013;39:1121–1127. doi: 10.1007/s00134-013-2827-x. [DOI] [PubMed] [Google Scholar]
- 3. Richard J-CM, Maggiore SM, Mancebo J, Lemaire F, Jonson B, Brochard L. Effects of vertical positioning on gas exchange and lung volumes in acute respiratory distress syndrome. Intensive Care Med . 2006;32:1623–1626. doi: 10.1007/s00134-006-0299-y. [DOI] [PubMed] [Google Scholar]
- 4. Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, et al. PLUG Working Group (Acute Respiratory Failure Section of the European Society of Intensive Care Medicine) The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med . 2014;189:520–531. doi: 10.1164/rccm.201312-2193CI. [DOI] [PubMed] [Google Scholar]
- 5. Chen L, Del Sorbo L, Grieco DL, Junhasavasdikul D, Rittayamai N, Soliman I, et al. Potential for lung recruitment estimated by the recruitment-to-inflation ratio in acute respiratory distress syndrome: a clinical trial. Am J Respir Crit Care Med . 2020;201:178–187. doi: 10.1164/rccm.201902-0334OC. [DOI] [PubMed] [Google Scholar]
- 6. Rezoagli E, Bastia L, Grassi A, Chieregato A, Langer T, Grasselli G, et al. Paradoxical effect of chest wall compression on respiratory system compliance: a multicenter case series of patients with ARDS, with multimodal assessment. Chest . 2021;160:1335–1339. doi: 10.1016/j.chest.2021.05.057. [DOI] [PubMed] [Google Scholar]
- 7. Spooner AJ, Corley A, Sharpe NA, Barnett AG, Caruana LR, Hammond NE, et al. Head-of-bed elevation improves end-expiratory lung volumes in mechanically ventilated subjects: a prospective observational study. Respir Care . 2014;59:1583–1589. doi: 10.4187/respcare.02733. [DOI] [PubMed] [Google Scholar]
- 8. Chiumello D, Carlesso E, Cadringher P, Caironi P, Valenza F, Polli F, et al. Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med . 2008;178:346–355. doi: 10.1164/rccm.200710-1589OC. [DOI] [PubMed] [Google Scholar]
- 9. Gattinoni L, Tonetti T, Cressoni M, Cadringher P, Herrmann P, Moerer O, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med . 2016;42:1567–1575. doi: 10.1007/s00134-016-4505-2. [DOI] [PubMed] [Google Scholar]
- 10. Yoshida T, Amato MBP, Grieco DL, Chen L, Lima CAS, Roldan R, et al. Esophageal manometry and regional transpulmonary pressure in lung injury. Am J Respir Crit Care Med . 2018;197:1018–1026. doi: 10.1164/rccm.201709-1806OC. [DOI] [PubMed] [Google Scholar]