Figure 1.
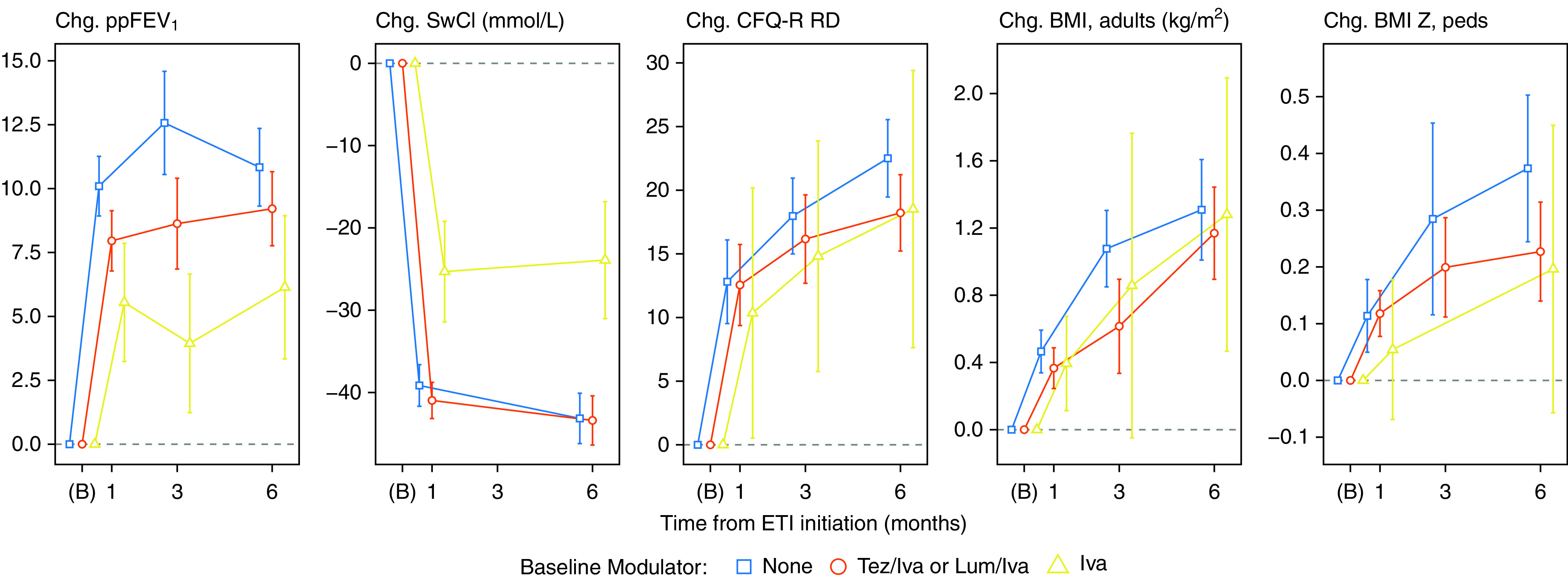
Changes from baseline with 95% confidence intervals, stratified by cystic fibrosis transmembrane conductance regulator modulator use at baseline (iva = ivacaftor monotherapy). Times of observation are pre-elaxaftor/tezacaftor/ivacaftor baseline (B) and planned visit times. Participants who were pregnant at a visit were excluded from analyses of body mass index (BMI). Confidence intervals with five or fewer participants are not shown, and low follow-up at 3 months requires additional caution in interpretation (see Table 2). CFQ-R RD = Cystic Fibrosis Questionnaire–Revised, Respiratory Domain; Chg. = change; ETI = elaxaftor/tezacaftor/ivacaftor; Lum = lumacaftor; ppFEV1 = percent predicted FEV1; SwCl = sweat chloride; Tez = tezacaftor.
