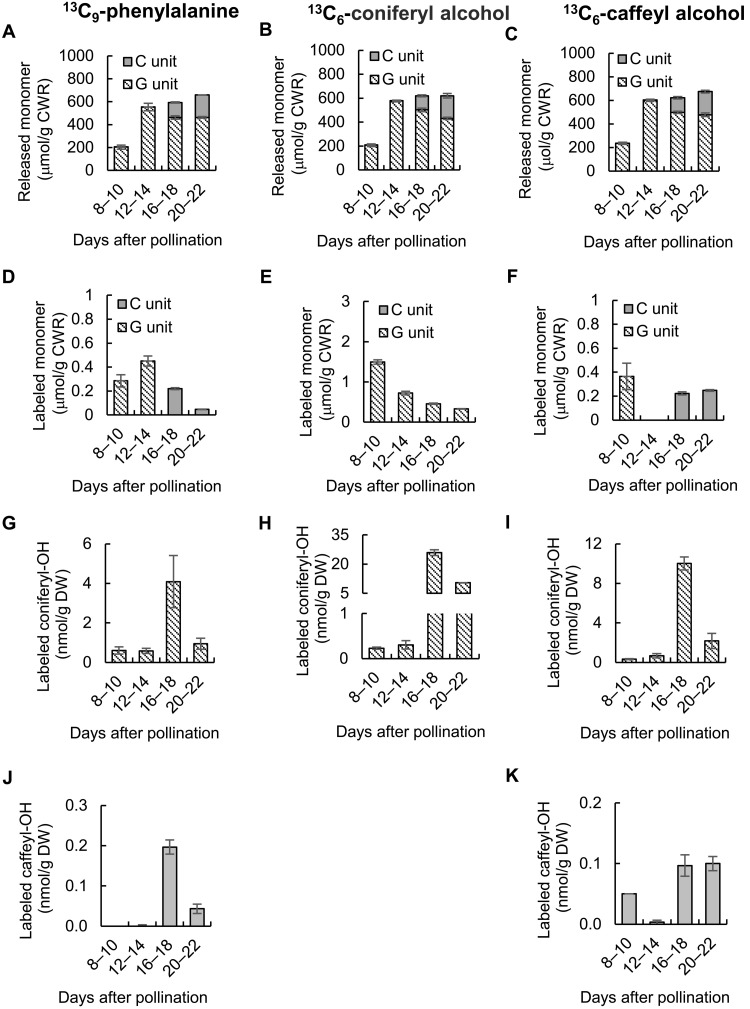
Fig. 3. Incorporation of 13C–l-phenylalanine, 13C-coniferyl alcohol, and 13C-caffeyl alcohol into lignin and monolignol pools in developing seed coats of C. hassleriana.
(A to C) Thioacidolysis yields of total G- and C-monolignols from lignin isolated from Cleome seed coats fed 13C-Phe (A), 13C-coniferyl alcohol (B), or 13C-caffeyl alcohol (C) at the times shown. (D to F) Thioacidolysis yields of 13C-labeled G- and C-monolignols from lignin isolated from Cleome seed coats fed 13C-Phe (D), 13C-coniferyl alcohol (E), or 13C-caffeyl alcohol (F) at the times shown. (G to I) 13C-labeled coniferyl alcohol extracted from Cleome seed coats fed 13C-Phe (G), 13C-coniferyl alcohol (H), or 13C-caffeyl alcohol (I) at the times shown. (J and K) 13C-labeled caffeyl alcohol extracted from Cleome seed coats fed 13C-Phe (J) or 13C-caffeyl alcohol (K) at the times shown. Amounts of labeled monomers in cellular pools, or as components of lignin, were calculated for the M + 7 ions (13C-Phe) or M + 6 ions (13C-coniferyl alcohol and 13C-caffeyl alcohol) by the method described previously (12). Approximately 100 mg of seed coats isolated from seeds harvested from each Cleome plant were counted as one biological replicate. Data are means ± SE derived from three biological replicates.