Abstract
OBJECTIVES
Left ventricular assist device (LVAD) implantation for end-stage heart failure patients has been on the rise, providing a reliable long-term option. For some LVAD patients, longer term LV unloading leads to recovery; hence, the need for evaluating potential myocardial recovery and weaning eligibility has emerged.
METHODS
All patients who underwent contemporary LVAD explantation at our institution between 2009 and 2020 were included in the study. Patients in New York Heart Association I, left ventricular ejection fraction >40%, a cardiac index >2.4 l/min and a peak oxygen intake >50% predicted underwent a 4-phase weaning assessment. A minimally invasive approach using a titanium plug was the surgery of choice in the most recent explants. Kaplan–Meier curves were used to estimate the survival at 1 and 5 years.
RESULTS
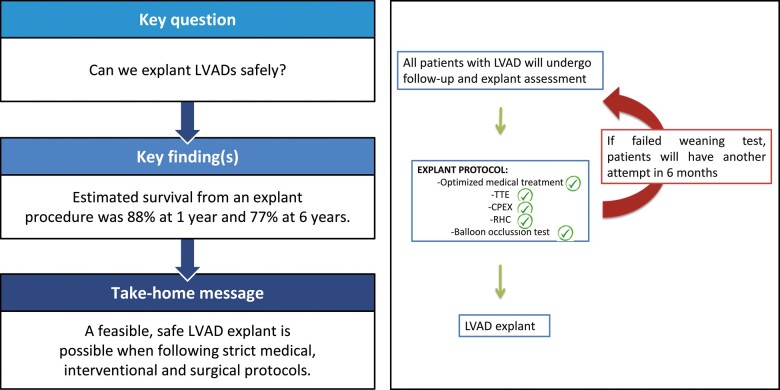
Twenty-six patients (17 HeartMate II, 9 HeartWare) underwent LVAD explantation after a median 317 days of support [IQ (212–518)], range 131–1437. Mean age at explant was 35.8 ± 12.7 years and 85% were males. Idiopathic dilated cardiomyopathy was the underlying diagnosis in 70% of cases. Thirteen (48%) patients were on short-term mechanical circulatory support and 60% required intensive care unit admission prior to the LVAD implantation. At 1 year, Kaplan–Meier estimated survival was 88%, whereas at 6 years, it was 77%. The average left ventricular ejection fraction at 1 year post-explant was 44.25% ± 8.44.
CONCLUSIONS
The use of a standardized weaning protocol (echocardiographic and invasive) and a minimally invasive LVAD explant technique minimizes periprocedural complications and leads to good long-term device-free survival rates.
Keywords: Left ventricular assist device, Myocardial recovery, Minimal invasive approach, Occlusion test, Left ventricular assist device explantation
Physicians are faced with a prevalence of >25 million heart failure (HF) cases worldwide [1].
INTRODUCTION
Physicians are faced with a prevalence of >25 million heart failure (HF) cases worldwide [1]. The incidence of HF, as well as survival with the disease, is increasing, resulting in an increased prevalence [2]. Heart transplantation (HTx) remains the gold standard for the treatment of end-stage HF; however, shortage of donor organs and contraindications have driven efforts to develop long-term mechanical circulatory support (MCS) alternatives. Left ventricular assist device (LVAD) implantation rates have increased over the last decade, providing good outcomes either as bridge to transplantation or as destination therapy demonstrating improved survival compared to medical therapy alone [3].
An LVAD allows for optimal titration of HF medication, which subsequently increases the chances of myocardial recovery [4, 5]. According to the fifth INTERMACS report, 1.2% of patients with LVAD were bridged to recovery [6]. It is estimated that 5% of ischaemic and 21% of non-ischaemic cardiomyopathies experience some degree of ventricular recovery when unloading the LV with LVAD [7]. In some cases, this may be significant enough to allow for weaning and explantation [8]. Peripartum cardiomyopathy [9] is associated with a higher probability of myocardial recovery and has shown better outcomes compared to other HF aetiologies [10].
Different explantation strategies have been described in the literature from decommissioning techniques leaving the pump in situ and ligating the graft, to cutting the driveline, or even full explantation of the pump, with ventriculoplasty and removal of the sewing ring [11].
Evaluation for possible myocardial recovery should be an integral part of care protocols in centres with MCS [12]. To date, there are no generally accepted criteria for LVAD weaning or explantation, and no prospective studies have been performed [13]. In the current paper, we describe in detail our LVAD weaning protocol and a minimally invasive surgical explant approach.
METHODS
We reviewed all contemporary LVAD patients who met criteria for explantation in Harefield Hospital, Royal Brompton and Harefield NHS Foundation Trust, London, UK. Out of 470 patients, we identified 52 long-term LVAD patients who underwent device explantation.
In the current study, we only included patients with contemporary devices such as HeartMate II™ (Abbott, Abbott Laboratories, Abbott Park, IL) or HeartWare™ HVAD™ (Medtronic, Minneapolis, MN). We identified a total of 26 patients who underwent contemporary LVAD explantation between January 2009 and February 2020 (Fig. 1).
Figure 1:

Inclusion criteria. LVAD: left ventricular assist device.
Ethical statement
Our local Institutional Review Board approved this work for submission and publication and advised that no specific informed consent was required due to the retrospective nature of the study and the use of fully anonymized data, which are part of an ongoing audit.
Standardized screening protocol
All the patients who undergo an LVAD implantation in our institution are commenced on guideline-directed medical therapy for HF (beta blockers/angiotensin-converting enzyme inhibitors/angiotensin II receptor blocker/angiotensin receptor neprilysin inhibitor and aldosterone antagonists), the doses of which are increased to the maximum tolerated. The routine follow-up protocol includes an echocardiogram on a 3-monthly basis, a cardiopulmonary exercise test every 6 months, a right-heart catheterization (RHC) once a year and blood tests with biomarkers such as brain natriuretic peptide (brain natriuretic peptide/NT-pro-brain natriuretic peptide) at each clinic visit. These tests allow us to identify patients who exhibit signs of recovery.
As initial inclusion criteria, we selected those patients who were in New York Heart Association class I, who had an ejection fraction >40%, a cardiac index >2.4 l/min and a peak oxygen intake >50% predicted.
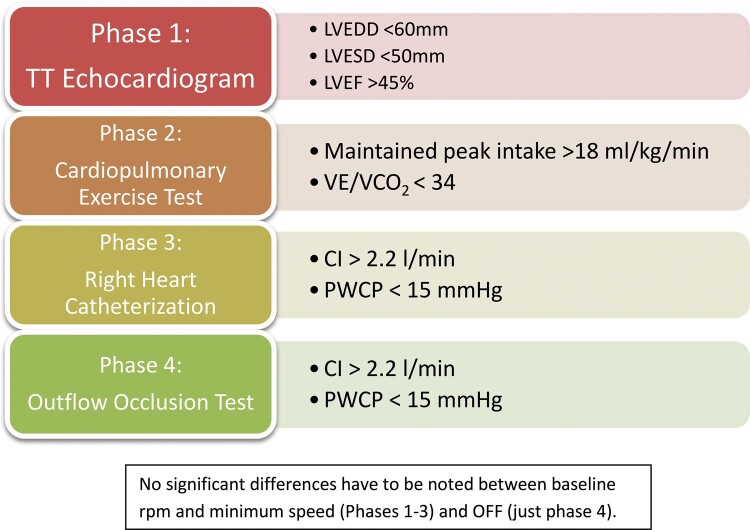
Selected patients subsequently underwent the 4-phase process detailed below (Fig. 2). The INR is determined the same day of the test: if it is ˂2, we do not proceed; if it is between 2 and 2.5, a bolus of 10.000 units of heparin is given; if it is between 2.5 and 3, a bolus of 5000 units is administered; and if it is >3, there is no need for extra anticoagulation.
Figure 2:
Scheme of medical and interventional protocol. CI: cardiac index; LVEDD: left ventricular end-diastolic diameter; LVESD: left ventricular end-systolic diameter; LVEF: left ventricular ejection fraction; PCWP: pulmonary capillary wedge pressure; VE/VCO2: minute ventilation relative to carbon dioxide production.
First, an echocardiogram is performed at 2 different speeds: baseline (9000 rpm and 2700 rpm for HeartMate II and HeartWare, respectively) and at 6000 rpm (HMII) and 1800 rpm (HVAD), each of those separated by a 10-min interval. The following measurements are recorded: the left ventricular end-diastolic diameter (LVEDD) and left ventricular end-systolic diameter (LVESD) in the parasternal view, the left ventricular ejection fraction and the degree of mitral regurgitation subjectively evaluated by an experienced echocardiographer. Following the criteria used in the RESTAGE trial [14], the patient would be considered suitable for LVAD explant if the LVEDD is lower than 60 mm, the LVESD lower than 50 mm and the left ventricular ejection fraction >45% without experiencing a significant change when the speed was decreased. Patients fulfilling these criteria would proceed to phase 2.
On a different day, a cardiopulmonary exercise is carried out at the same speeds mentioned above. Those who maintained a peak oxygen intake >18 ml/Kg/min and a minute ventilation relative to carbon dioxide production (VE/VCO2)<34 moved on to phase 3.
The third stage includes an RHC. All the routine measurements are recorded at baseline speed and at 6000 rpm (HMII)/1800 rpm (HVAD). The test was considered positive if the cardiac index remained >2.2 l/min and the pulmonary wedge pressure did not increase >15 mmHg at the reduced speed.
If these 3 steps were considered satisfactory, the patient was scheduled to have the outflow occlusion test described below. This procedure was usually arranged on the day of surgery, meaning that the patient was intubated allowing for simultaneous transoesophageal echocardiography.
Cath lab occlusion test
RHC parameters are assessed in 3 stages.
With LVAD at full flow, i.e. 2700 rpm (HVAD), or 9000 rpm (HM II)
At the lowest LVAD speed, i.e. 1800 rpm (HVAD), 6000 rpm (HM II)
With LVAD switched off and the outflow graft occluded for 5 min
Invasive haemodynamics are measured at the level of the right atrium, right ventricle, pulmonary artery, pulmonary capillary wedge pressure and O2 saturations were obtained at the level of the right atrium and the PA.
A total of 100 units/kg of heparin were given aiming for an Activated Clotting Time (ACT) 250–300 s. The outflow graft was engaged using a multipurpose 6 Fr diagnostic catheter and the J wire was advanced deep to the proximal part of the outflow graft. The multipurpose catheter would then be advanced, and the J wire exchanged for an Amplatz super stiff wire. For stage 3 of the procedure, an Abbott Armada™ 10 mm × 40 mm balloon is advanced retrogradely over the super stiff wire to the mid-segment of the outflow graft and inflated to 2 atm for 5 min. This manoeuvre is essential to avoid acute LV overload and decompensation secondary to regurgitation of blood from the aorta to the LV via the graft.
The criteria used to determine whether the explant could be performed were the same as for the initial RHC: cardiac index >2.2 l/min and pulmonary capillary wedge pressure <15 mmHg.
Surgical protocol
In recent years, the majority of our patients have undergone LVAD implantation via bilateral thoracotomy on cardiopulmonary bypass (CPB) via femoral cannulation.
The apical coring of myocardial tissue retrieved during implantation is always sent to histology to identify or confirm the underlying pathology.
Pre-explant surgical planning includes computed tomography of the thorax, abdomen and femoral vessels in order to assess the LVAD and outflow graft position as well as the trajectory of the driveline and the suitability of the femoral vessels for cannulation.
Warfarin is discontinued 3 days before surgery and heparin infusion is initiated if the INR falls ˂2. Intraoperatively, patients are intubated with a dual lumen tube to allow single-lung ventilation. Transoesophageal echocardiography and placement of a Swan Ganz catheter are routine practices.
Patients undergo a redo or neo left anterolateral thoracotomy. This provides good access to both the pump and the driveline.
Once the device is fully exposed, CPB is initiated via femoral cannulation and CO2 is instilled in the operative field. The outflow graft and the driveline are exposed. A final occlusion test is performed by temporary clamping of the outflow graft. The outflow is clamped and distally secured with a holding suture to prevent retraction. It is then divided and oversewn distally. The driveline is cut and mobilized.
CPB drainage and flow are increased to reduce ejection to the minimum and the holding screw is loosened on the ring. In the majority of cases implanted with an HVAD, intraventricular thrombotic pannus can be found surrounding the insertion site. As we have never been able to remove this material from any of the patients and given that no patient has experienced a Cerebrovascular accident (CVA) to date, we have stopped routinely inspecting the insertion site and now directly insert a custom-made titanium plug. This plug is manufactured by Steffan Fittkau GmbH, a metallurgy and engineering firm in Berlin, Germany, and has been adopted as an off-label procedure and tool for VAD removal (Fig. 3). In patients with HeartMate II device, the apical insertion site is oversewn, as there are no plugs available for this manufacturer.
Figure 3:
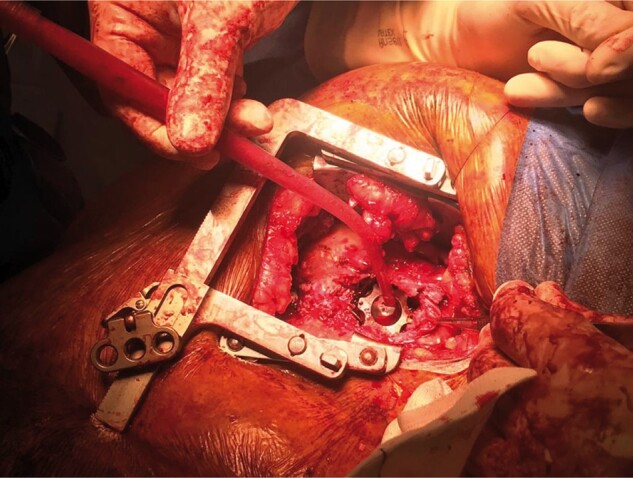
Plug placed in the left ventricle.
Anticoagulation is reversed with Protamine sulphate at the end of the case once the patient is stable.
Following the surgery, all patients continue or up-titrate their conventional HF treatment. Patients continue to be followed up in the advanced HF clinic, undergoing routine monitoring.
All patients undergoing LVAD explant are anticoagulated for at least 6 months aiming for an INR target of 2–3 in the absence of contraindications.
Statistical analysis
All continuous variables were tested for normality using the Kolmogorov–Smirnov test. Data are presented as percentages, mean ± standard deviation or median (interquartile range). Differences in proportions were tested with χ2 test or Fisher’s exact test, and differences in continuous variables were tested with Student’s paired t-test or Wilcoxon signed-rank sum test for parametric and nonparametric variables, respectively. The Kaplan–Meier estimate was used to calculate 1- and 5-year survival post-explant.
RESULTS
Patients’ pre-left ventricular assist device characteristics
Diagnosis at the time of implant is shown in Table 1 with 70% of the patients having idiopathic dilated cardiomyopathy. The mean age of implant was 35.8 years [range (16–58)] and 84% of the patients were male.
Table 1:
Implant demographics
| Variables | N = 26 (%) |
|---|---|
| Age (years), mean ± SD (range) | 35.8 ± 12.7 (16–58) |
| Male gender | 22 (84) |
| Diagnosis | 18 DCM; 4 myocarditis; 2 ICM; 2 postpartum CM |
| Duration of chronic heart failure (months), mean ± SD | 5.6 ± 1.2 |
| MCS pre implant | 4 IABP; 4 ECMO; 5 Levitronix |
| Intensive care unit admission | 16 (69) |
| Orotracheal intubation required | 8 (30) |
| Inotropic support | 24 (89) |
| Type of device | 17 (65) HeartMate/9 (34) HeartWare |
| Surgical approach of implant | 23 (88) sternotomy/3 (11.5) bilateral thoracotomy |
DCM: dilated cardiomyopathy; ECMO: extracorporeal membrane oxygenation; IABP: Intraaortic Balloon Pump; MCS: mechanical circulatory support; SD: standard deviation.
Of the patients, 48.1% [13] were on short-term MCS prior to the LVAD implantation. The type of device is described in Table 1. Sixty per cent of patients required intensive care unit admission prior to LVAD implantation. Thirty per cent were intubated and 89% required inotropic support.
Eighty-eight per cent of patients underwent implantation of the LVAD via median sternotomy, whereas more recently, the bilateral thoracotomy approach was used. The latter is our preferred approach when there are no contraindications and no other concomitant procedures are necessary.
A total of 257 HeartWare and 80 HMII were implanted. This means 21% of the HMII as 3.5% of the HeartWare devices were explanted. It is important to highlight that since 2011 we solely implant HeartWare devices.
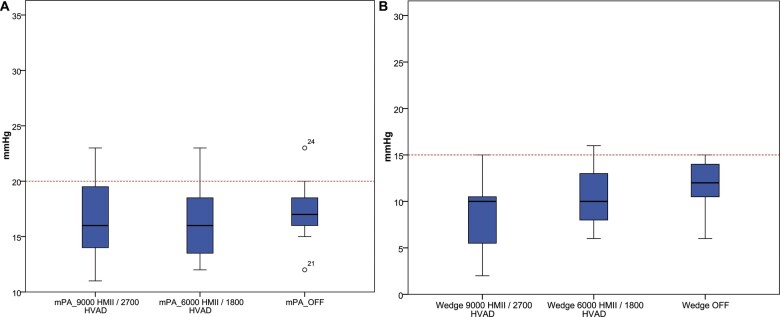
In Table 2, we show the comparisons RHC parameters. No significant changes were seen in haemodynamic parameters after reducing the speed of the LVAD or occluding the outflow graft and stopping the LVAD. This is graphically demonstrated in Fig. 4 where both mPA and pulmonary capillary wedge pressure did not exhibit a significant increase throughout the 3 phases of the occlusion test (full flow, minimum speed and off). No adverse events were noted during the occlusion test in any of the patients.
Table 2:
Variables compared regarding speed of LVAD
| Variables | Mean FF-minimum rpm |
P-value FF-minimum rpm |
Mean FF-OFF |
P-value FF-OFF |
|---|---|---|---|---|
| mPA, mean ± SD | 0.3 ± 1.2 | 0.539 | −0.6 ± 3.2 | 0.904 |
| Wedge, mean ± SD | −2.1 ± 2.5 | 0.139 | −3.3 ± 3.9 | 0.061 |
| Cardiac output, mean ± SD | 0.02 ± 0.7 | 0.928 | 0.1 ± 3 | 0.179 |
| Cardiac index, mean ± SD | 0.03 ± 0.3 | 0.859 | 0.09 ± 1.4 | 0.121 |
FF: full flow of the LVAD; LVAD: left ventricular assist device; Minimum rpm: minimum speed (HMII 6000 rpm, HVAD 1800 rpm); mPA: mean pulmonary pressure; SD: standard deviation.
Figure 4:
Comparison between mean pulmonary artery pressures and wedge with full flow LVAD, minimum speed and LVAD off and occluded. LVAD: left ventricular assist device.
Explantation
Seventeen HeartMate II patients and 9 HeartWare underwent explantation of the device. The median age at the time of explant was 37 ± 12 years [range (17–58)]. Median duration of support was 317 days [Interquartile (IQ) (212–518)]. When analysing the last 7 cases, the average duration of support was 713 ± 472 days. The longest duration of LVAD support was 1427 days in a 54-year-old patient with dilated cardiomyopathy, whereas the minimum duration of support was 131 days in a 31-year-old male with dilated cardiomyopathy.
LVESD and LVEDD were 3.54 ± 0.63 cm and 4.89 ± 0.62 cm, respectively, on postoperative echocardiography.
As shown in Table 3, the explant approach has evolved with the time. At the beginning of the study, all the explants were performed via a redo sternotomy. The opening left by the ring of the inflow graft was oversewn.
Table 3:
Explant surgical approach
| Surgical approach explanta | N = 26 (%) |
|---|---|
| Median sternotomy | 10 (38) |
| Left anterolateral thoracotomyb | 9 (34) |
| Bilateral thoracotomies | 4 (15) |
| Sternotomy and left thoracotomy | 3 (11.5) |
The only devices removed were HVAD and HeartMate II.
Seven patients had HeartWare and 2 had HeartMate II.
Later on, the explants were approached via bilateral thoracotomy or redo sternotomy and left thoracotomy to explant the inflow and cut and ligate the outflow graft.
The last 7 cases have been done solely via a left anterolateral thoracotomy.
Left anterolateral thoracotomy was performed in 77% of the HeartWare patients and in 12% of the HeartMate II patients (7 HeartWare and 2 HeartMate II patients).
Of the 26 explanted patients, only 1 patient required extracorporeal membrane oxygenation (ECMO) postoperatively and unfortunately died 14 days post-explant due to profound coagulopathy and multiorgan failure. The inotropes used in our department are at the discretion of the anaesthetic department, but mostly all patients came out of theatre on small doses of adrenaline and milrinone. The median CPB time was 34 min (interquartile range 14–69).
Follow-up
During a median follow-up time of 7 years (interquartile range 0.7–10.3), 9 patients died (34.6%). Two patients (7.7%) died in the early postoperative (in-hospital) period due to coagulopathy and multi-organ failure; one of them was placed on ECMO. One post-explant patient died after 3 years, as he was not an HTx candidate due to non-compliant behaviour. Another patient had a decompensation 10 years post-explant. We implanted an Impella 5.0 as bridge to a new LVAD; however, he never managed to recover from multi-organ failure. One patient died 10 years post-explant while on holidays abroad due to a sudden cardiac arrest. Two patients were transplanted 5 and 3 years post-explant, respectively, and died 2 and 7 years later due to acute rejection. One died 12 years post-explant following massive haematemesis. One patient had a new implant 4 years post original explant and died of pump thrombosis. All of the last 7 patients are alive.
One-year Kaplan–Meier estimated survival from explant is 87.9%. Survival at 6 years from explant is 77.8% (Fig. 5).
Figure 5:
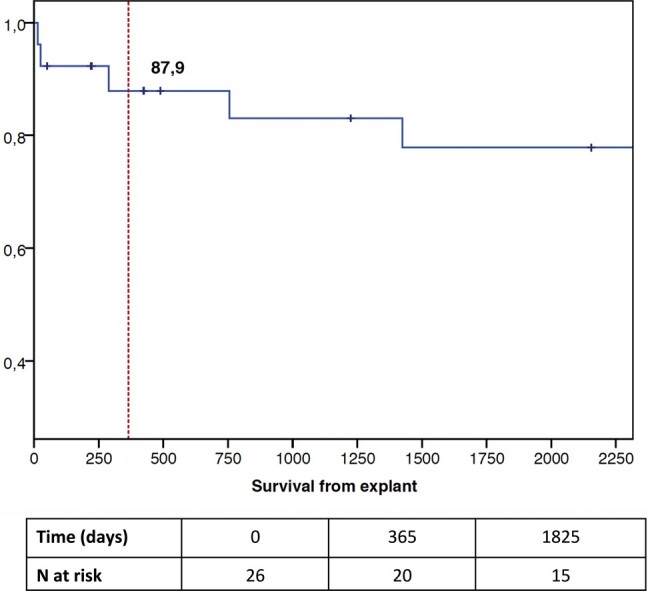
Survival from explant shown in days.
The follow-up average ejection fraction at 1 year post-explant is 44.25% ± 8.44, while LVESD and LVEDD are 4.54 ± 0.68 and 5.8 ± 0.46 cm, respectively.
DISCUSSION
In the current study, we show good long-term outcomes after LVAD explantation in patients who pass a strict 4-phase weaning protocol. If validated in future studies, this approach could become the gold standard for patients with LVAD who demonstrate improving left ventricular ejection fraction over time due to LV unloading.
HTx is the gold standard treatment for end-stage HF. However, we are facing a continuous shortage of donors and a change in donor profile; this, paralleled by improvements in device therapy, has resulted in a surge in the numbers of LVAD implantation. Consequently, the number of patients with LVADs who recover significant LV function in the long run will increase, making weaning and explantation of LVAD the best therapeutic outcome and the most desirable goal.
Approximately 1–2% of patients with LVAD in the global literature [15] will have sufficient myocardial recovery to allow for explantation. Our explant rates in the current study were higher (∼11%), but this was mainly due to the lack of availability of percutaneous or minimally invasive short-term MCS (such as Impella family of devices) up to 4 years ago. Even though patients with a long history of HF can recover some function, as shown by Birks et al. [16], patients with shorter history of HF had a higher rate of myocardial recovery. We report an average of 317 days [IQ (212–518)] of support prior to explant. When analysing the last 7 cases, the average duration of support was 713 ± 472 days. Pan et al. [17] identified younger age, female sex, lower body index, non-ischaemic cause and shorter duration of HF before implantation as predictors of myocardial recovery.
As reported in the latest INTERMACS report [18], patients with higher INTERMACS profiles have significantly increased risk for early mortality. Following stabilization with percutaneous MCS, early LVAD implantation, before irreversible damage and remodelling occurs, could result in more patients recovering myocardial function. In such high-risk cases, implantation of an LVAD is the only option, as these patients are either too unwell to wait or not eligible for HTx at that point in time. For those patients who are on long-term support, LVAD explantation can be the only valid option for those who experience associated MCS complications and are not eligible for HTx. If recovery could be instigated and controlled in this cohort of patients, it would, of course, make the long-term difference. As the study spans over a long period of time, all patients before 2010 with the exception of those with myocarditis were treated with Clembuterol as per RESTAGE trial [14]. From 2010 onwards, we have not used Clembuterol in any patient.
Jakovljevic et al. [4] report better cardiac and functional capacity in patients with explanted LVAD compared to those with LVADs or even heart transplant patients, with peak oxygen consumption similar to healthy controls. In line with their study, we report good 1- and 5-year survival rates at 88% and 78%, respectively.
Regarding surgical techniques, Baldwin et al. [19] describe 4 different methods of device removal or decommissioning. Their survival analysis showed no association between surgical approach and survival. However, ring removal and plication of the coring site have been shown to cause a significant deformation of the LV geometry [20]. Also, it makes reinsertion of a device more difficult, should the need arise.
Kapoor et al. [8] describe the surgical approach of a right anterior mini thoracotomy to ligate the outflow graft and remove the driveline leaving the pump in situ. Resternotomy has been described as an independent risk factor for cardiac injury. Indeed, while this avoids the surgical risks of a resternotomy, the infection and thrombosis risks of leaving the pump in situ may be significant. In this study, patients were only on long-term aspirin as sole antithrombotic therapy.
Decommissioning techniques like ligation of the outflow graft or cutting the driveline might be appropriate for frail patients with high surgical risk [21]; however, this approach presents risks of thromboembolism and infection by leaving the VAD in situ. This necessitates lifelong anticoagulation, as well as it might affect the myocardial recovery and should not be the standard approach for patients with device infection.
In our institution, we always aim for a minimally invasive surgical approach via femoral cannulation to initiate the CPB without necessitating cardiac arrest. Using a left anterolateral thoracotomy, we avoid re- or neo-sternotomy, bleeding, infections and right ventricular failure, as we do not access the pericardium. With this approach, we not only avoid the risk of infection by removing the pump but also the risk of thrombosis. The driveline is fully removed and its exit point at the skin level is debrided. The use of the plug ensures that LV geometry is preserved, which might further aid long-term recovery and avoids the risk of thrombosis and strokes. Furthermore, it allows for a faster reimplantation of the LVAD if needed, as the sewing ring remains in place. The plug inserted in the sewing ring is a custom-made piece [22] made of titanium alloy, the diameter of which matches that of the sewing ring. It can be implanted via the left thoracotomy with a special plug-holder device. This technique ameliorates resternotomy risks, and is associated with less bleeding and lower need for transfusions [11]. The plug is not CE-marked and can only be used as a compassionate device in most countries. All patients need to be anticoagulated for at least 6 months.
Limitations
In our study, we report the explant protocol and outcomes of a small cohort of patients with previous LVAD implantation. One of the major limitations has to do with the small sample size. Furthermore, the long span of the study makes the sample rather heterogeneous from every perspective (aetiology of HF, type of device implanted and surgical explantation technique); hence, conclusions can only be speculative. Over a period of 10 years, the implant and explant surgical techniques have evolved due to growing experience. It should be highlighted that the medical, interventional and surgical protocol described has been current practice for the last 7 years. Another limitation of the current study is the absence of comparison data between successfully explanted and not-explanted patients, which could have shed more light on the eligibility criteria. Given the strict entry criteria, however, the vast majority of cases put forward for explant assessment do make it to the procedure.
CONCLUSIONS
In the current paper, we present a detailed 4-stage weaning protocol for LVAD patients who have recovered a degree of LV systolic function. We have shown a good 5-year survival post-explant when this strict weaning protocol is followed by a minimally invasive surgical explant. Given the increasing incidence and prevalence of end-stage HF and the rising number of LVADs, standardized guidelines and protocols for explantation are invaluable, as the number of patients with potential myocardial recovery might increase in the upcoming years.
Abbreviations
- CPB
Cardiopulmonary bypass
- ECMO
Extracorporeal membrane oxygenation
- HF
Heart failure
- LVAD
Left ventricular assist device
- LVEDD
Left ventricular end-diastolic diameter
- LVESD
Left ventricular end-systolic diameter
- MCS
Mechanical circulatory support
- RHC
Right-heart catheterization
Conflict of interest: none declared.
Author contributions
María Monteagudo Vela: Conceptualization; Data curation; Formal analysis; Writing—original draft; Writing—review & editing. Verónica Rial Bastón: Data curation; Writing—review & editing. Vasileios Panoulas: Data curation; Formal analysis; Writing—review & editing. Fernando Riesgo Gil: Data curation; Writing—review & editing. Andre Simon: Validation; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Stefano Mastrobuoni, Davide Pacini, David Spielvogel and the other, anonymous reviewer(s) for their contribution to the peer-review process of this article.
REFERENCES
- 1. Roger VL. Epidemiology of heart failure. Circ Res 2013;113:646–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 2020;141:e139–596. [DOI] [PubMed] [Google Scholar]
- 3. Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241–51. [DOI] [PubMed] [Google Scholar]
- 4. Jakovljevic DG, Yacoub MH, Schueler S, MacGowan GA, Velicki L, Seferovic PM et al. Left ventricular assist device as a bridge to recovery for patients with advanced heart failure. J Am Coll Cardiol 2017;69:1924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Birks EJ, Tansley PD, Hardy J, George RS, Bowles CT, Burke M et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med 2006;355:1873–84. [DOI] [PubMed] [Google Scholar]
- 6. Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant 2013;32:141–56. [DOI] [PubMed] [Google Scholar]
- 7. Wever-Pinzon J, Selzman CH, Stoddard G, Wever-Pinzon O, Catino A, Kfoury AG et al. Impact of ischemic heart failure etiology on cardiac recovery during mechanical unloading. J Am Coll Cardiol 2016;68:1741–52. [DOI] [PubMed] [Google Scholar]
- 8. Kapoor K, Shah P, Bogar LJ, Edwards LG, Panjrath GS, Singh R. Long-term safety of minimally invasive left ventricular assist device discontinuation for myocardial recovery. Ann Thorac Surg 2019;108:1398–403. [DOI] [PubMed] [Google Scholar]
- 9. Lund LH, Grinnemo KH, Svenarud P, van der Linden J, Eriksson MJ. Myocardial recovery in peri-partum cardiomyopathy after continuous flow left ventricular assist device. J Cardiothorac Surg 2011;6:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cooper LT, Mather PJ, Alexis JD, Pauly DF, Torre-Amione G, Wittstein IS et al. Myocardial recovery in peripartum cardiomyopathy: prospective comparison with recent onset cardiomyopathy in men and nonperipartum women. J Card Fail 2012;18:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ricklefs M, Deodhar C, Chatterjee A, Feldmann C, Hanke JS, Heimeshoff J et al. A new tool for an explantation strategy of HeartMate 3 left ventricular assist device. J Thorac Dis 2018;10:S1825–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akil A, Fischer D, Holthaus AJ, Martens S, Scherer M, Sindermann JR. Left ventricular assist devices as bridge to cardiac recovery in nonischemic heart failure: keeping weaning from the device in mind. Thorac Cardiovasc Surg 2015;64:483–6. [DOI] [PubMed] [Google Scholar]
- 13. Selzman CH, Madden JL, Healy AH, McKellar SH, Koliopoulou A, Stehlik J et al. Bridge to removal: a paradigm shift for left ventricular assist device therapy. Ann Thorac Surg 2015;99:360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Birks EJ, Rame E, Patel SR. Remission from stage D heart failure (RESTAGE-HF): interim results and insights from a prospective multi-center non-randomized study of myocardial recovery using LVADs. Circulation 2016;134:A19838. [Google Scholar]
- 15. Antonides CFJ, Schoenrath F, de By T, Muslem R, Veen K, Yalcin YC et al. Outcomes of patients after successful left ventricular assist device explantation: a EUROMACS study. ESC Heart Fail 2020;7:1085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Birks EJ, George RS, Hedger M, Bahrami T, Wilton P, Bowles CT et al. Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy: a prospective study. Circulation 2011;123:381–90. [DOI] [PubMed] [Google Scholar]
- 17. Pan S, Aksut B, Wever-Pinzon OE, Rao SD, Levin AP, Garan AR et al. Incidence and predictors of myocardial recovery on long-term left ventricular assist device support: results from the United Network for Organ Sharing database. J Heart Lung Transplant 2015;34:1624–9. [DOI] [PubMed] [Google Scholar]
- 18. Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant 2017;36:1080–6. [DOI] [PubMed] [Google Scholar]
- 19. Baldwin AC, Sandoval E, Letsou GV, Mallidi HR, Cohn WE, Frazier OH. Surgical approach to continuous-flow left ventricular assist device explantation: a comparison of outcomes. J Thorac Cardiovasc Surg 2016;151:192–8. [DOI] [PubMed] [Google Scholar]
- 20. Lara DA, Jeewa A, Elias BA, McCullum EO, Denfield SW, Dreyer WJ et al. Titanium plug closure after HeartWare ventricular assist device explantation in a 15-year-old girl: first U.S. experience. Tex Heart Inst J 2017;44:66–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Potapov EV, Antonides C, Crespo-Leiro MG, Combes A, Farber G, Hannan MM et al. EACTS expert consensus on long-term mechanical circulatory support. Eur J Cardiothorac Surg 2019;56:230–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monteagudo-Vela M, Panoulas V, Riesgo-Gil F, Simon A. Surgical explant of a right ventricular assist device with sternum-sparing technique. Eur J Cardiothorac Surg 2020;58:193–5. [DOI] [PubMed] [Google Scholar]