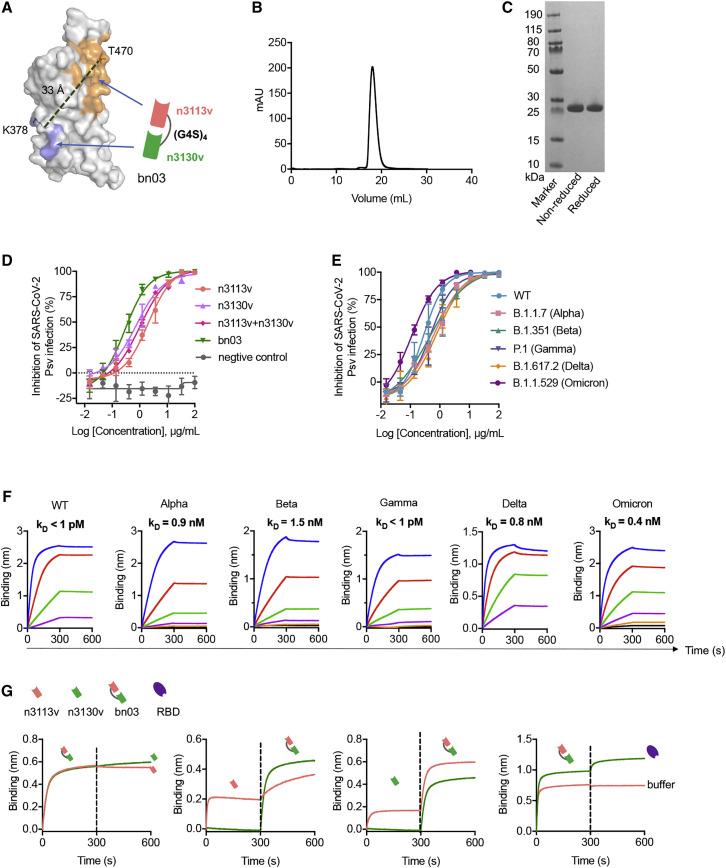
Figure 2.
The design of bispecific single-domain antibody bn03
(A) The bispecific single-domain antibody bn03 contains n3130v and n3113v linked with a linker (GGGGS)4. n3113v and n3130v are colored in pink and green, respectively. The RBD is depicted as gray surface with the epitopes of n3113v and CR3022 (targeting the same epitopes with n3130v) highlighted in orange and blue, respectively. K378 and T470 that are involved in the recognition of CR3022 and n3113, respectively, are shown as sticks. The distance between Cα of K378 and T470 was measured and is labeled in dashed green lines.
(B) Size exclusion chromatography profile of the bispecific antibody bn03.
(C) Reducing and non-reducing SDS-PAGE analysis of bn03.
(D) Neutralization of SARS-CoV-2 pseudoviruses by a panel of single-domain antibodies, including n3113v, n3130v, cocktail of n3113v and n3130v, and bn03. Three independent experiments were performed in triplicate.
(E) Neutralizing potency of bn03 against pseudoviruses of WT and five VOCs. Three independent experiments were performed in triplicate.
(F) Binding affinity of bn03 to RBDs of WT and five VOCs. The KD values are shown.
(G) The bispecific single-domain antibody bn03 simultaneously binds two distinct epitopes on the RBD. The immobilized RBD was incubated with bn03, n3113v (orange), or n3130v (green) until saturation and then incubated with second antibody or the RBD. The binding curves were monitored.
See also Figure S4.