Abstract
Adolescent and young adult (AYA) enrollment in cancer clinical trials (CCT) is suboptimal. Few studies have explored site level barriers and facilitators to AYA enrollment on CCTs and the efficacy of interventions to enhance enrollment. A cross sectional survey was developed by the COG AYA Oncology Discipline Committee Responsible Investigator (RI) Network to identify perceived barriers and facilitators to enrollment, as well as opportunities to improve enrollment. Associations of barriers and facilitators to enrollment with program demographics were assessed. The survey was sent to all AYA RI Network members (n = 143) and quantitative and thematic analyses were conducted. The overall response rate was 42% (n = 60/143). Participants represented diverse institutions based on size, presence or absence of dedicated AYA programs, and proximity and relationship between pediatric and medical oncology practices within the institution. The most frequently cited barriers to enrolling AYAs in CCTs were administrative logistical issues (45%), disparate enrollment practices (42%) and communication issues (27%) between pediatric and medical oncology and perceived limited trial availability (27%). The most frequently reported facilitators to enrollment included having strong communication between pediatric and medical oncology (48%), having a supportive research infrastructure (35%) and the presence of AYA champions (33%). Many barriers and facilitators were similar across institutions and AYA program types. Shared barriers and facilitators to AYA CCT enrollment exist across the landscape of cancer care settings. Interventions aimed at increasing coordination between pediatric and medical oncology clinical trials offices and providers have high potential to improve site-level AYA enrollment.
Subject terms: Health care, Oncology
Introduction
Cancer clinical trials (CCTs) are vital for studying disease biology and improving survival and health-related quality-of-life outcomes; however, only 2–5% of all AYAs with cancer enroll in a CCT1,2. Despite a growing focus on addressing disparities in AYA cancer care and outcomes, few studies have assessed factors contributing to the low enrollment of AYAs into CCTs. Even fewer studies have assessed the efficacy of interventions to improve enrollment. AYA cancer biology, tolerance to intensive treatment and survival outcomes for specific malignancies differ in comparison with older adults and younger children, strongly supporting the need to identify optimal treatment and supportive care approaches in the AYA population3,4.
The reasons for limited AYA CCT enrollment, even amongst the ones eligible for trials while not well understood, have been hypothesized in recent reviews5–7. These include global issues such as the perception of limited availability of relevant CCTs, regional issues such as lack of referral of AYA patients to centers with National Cancer Institute (NCI)-CCTs and institutional-level issues such as not activating CCTs due to the regulatory burden and cost of study activation and conduct. Additional suggested barriers at the site level include lack of eligibility screening procedures, limited communication between medical and pediatric oncologists, limited knowledge and comfort with other NCI Clinical Trials Network (NCTN)-CCTs, as well as time and economic constraints to open and enroll AYAs on CCTs. In addition psychosocial barriers such as stress/distress, developmental and emotional maturity, feeling ill, experimentation play a role as well8–12. While many publications have proposed potential barriers to enrollment, data reporting actual barriers are sparse8–12.
In 2018, the Children’s Oncology Group (COG) AYA Oncology Discipline Committee developed an international network of AYA Responsible Investigators (RIs) consisting of > 140 individuals from demographically and geographically diverse sites that serve a variety of distinct roles at their respective institutions such as physicians, nurse practitioners, nurse navigators and research staff. Institutions included free-standing children's hospitals, sites with pediatric and medical oncology on shared or separate campuses, and sites located in community and urban settings.
The primary purpose of the AYA RIs is to optimize AYA enrollment onto COG-led trials, and other NCTN trials in which COG is participating, at their sites. AYA RI responsibilities are focused on implementing steps to facilitate clinical trial enrollment of AYAs treated within their institution as previously described13. The primary mechanism through which the AYA RI Network supports enrollment is providing education and peer support to institutional AYA RIs. To achieve its goal, the AYA RI Network hosted a series of informal webinars allowing RIs to share barriers and facilitators to AYA enrollment, as well as unique challenges to AYA enrollment based on site-specific factors. The purpose of this survey was to systematically identify shared barriers and facilitators to AYA accrual in CCTs as reported by members of the COG AYA RI Network and assess associations with program demographics to inform institutional and national interventions aimed at increasing AYA CCT enrollment.
Methods
Survey design, development, and setting
A cross sectional survey was developed by the COG AYA Oncology Discipline Committee RI Network leadership (AS, NM, MR, DF) to identify perceived barriers and facilitators to enrollment, as well as opportunities to improve enrollment. The survey was piloted with five AYA oncology stakeholders and revised as needed for content, readability and clarity. The survey consisted of two parts: the first comprised twelve questions on demographic information, including program characteristics, institutional structure and the relationship between pediatric and medical oncologists; the second comprised four free text questions on participants’ perspective on: (1) institutional barriers to enrollment; (2) facilitators to enrollment; and (3) possible solutions to improve AYA accrual to CCTs at the institutional level; and (4) cooperative group level. Of note, participants could have their responses placed into multiple categories and multiple answers were permitted (Supplemental Table 1).
Survey distribution
The online survey was administered and responses were stored using REDCap. An email was sent to all COG AYA RI Network members (n = 143) with a brief description of the study and an embedded, clickable link to the survey. One RI from each institution in the RI Network was sent the survey. In case of non-response, the survey was redistributed two additional times within 4 weeks from initial survey distribution (December 2019–January 2020). All methods were carried out in accordance with relevant guidelines and regulations. The study was approved as exempt by the Prisma Health Upstate IRB. Participation in the survey was voluntary and signed informed consent was not obtained for individual participants as it would be the only link between the participant responses and their identity.
Data analysis
Demographic information was summarized for all respondents. Response frequencies and proportion of total responses were calculated for all categorical variables. Means and medians were calculated for all continuous variables. Responses to the free text questions were reviewed and themes for each of the four free text questions were identified by two study investigators (NM, AS). The themes were reviewed and consensus was achieved among three study investigators (NM, AS, MR). For each question, responses were subsequently independently categorized into the previously agreed upon themes (NM, AS). When there was disagreement in the categorization of responses, agreement was sought between the two raters (NM, AS); if consensus was not reached, a third, independent investigator (MR) categorized the response. Fisher exact test and Chi Square analysis (GraphPad Prism, San Diego, CA) were used to assess the association between demographic variables and perceived barriers and facilitators to enrollment. Two-sided p value < 0.05 was considered statistically significant.
Results
Respondent and institutional demographics
The overall survey response rate was 42% (n = 60/143) and 97% of these respondents (n = 58) completed the entire survey. The participants represented a diverse group of institutions based on size, presence of an AYA Program and geographic proximity between pediatric and medical oncology (Table 1). Approximately one-third (n = 22) of respondents reported that their institution saw > 100 new AYA patients annually. The percent of respondents that categorized their institution as a children’s hospital within an adult medical center, free standing children’s hospital, and community hospital was 50% (n = 29), 36% (n = 21) and 12% (n = 7), respectively.
Table 1.
Institutional and AYA program characteristics.
| Question | Response | N (% of responders) |
|---|---|---|
| AYA program existence | Yes/in development | 45 (75%) |
| No | 15 (25%) | |
| New AYA patients/yr at institution | < 100 | 38 (63%) |
| > 100 | 22 (37%) | |
| AYA program size (new patients/yr in past 3 yrs) | < 100 | 17 (57%) |
| > 100 | 13 (43%) | |
| AYA services provided | Cancer treatment | 23 (77%) |
| Genetic counseling | 18 (60%) | |
| Oncofertility | 29 (97%) | |
| Psychosocial support | 28 (93%) | |
| Sexual health | 15 (50%) | |
| Survivorship care | 21 (70%) | |
| Symptom management | 20 (67%) | |
| Cancer type served | All cancers | 26 (87%) |
| Limited | 4 (13%) | |
| Institution type | Free standing CH | 21 (36%) |
| CH within adult medical center | 29 (50%) | |
| Community hospital | 7 (12%) | |
| Academic medical center | 18 (31%) | |
| Geographic proximity (Ped/Med oncology) | Same building | 14 (24%) |
| Same campus | 28 (48%) | |
| Different campus | 11 (19%) | |
| Single institutional IRB | Yes | 43 (74%) |
| No | 12 (21%) | |
| Joint tumor boards | Yes (all/some diagnosis) | 25 (43%) |
| No | 33 (57%) | |
| Cross-department ad hoc AYA Discussions | Yes | 52 (90%) |
| No | 6 (10%) | |
| Med oncology enroll onto COG trials | Frequently/occasionally | 26 (45%) |
| Rarely/never | 24 (41%) | |
| No Med oncology at institution | 8 (14%) |
AYA program demographics
Of the 60 responders, three quarters reported having an active AYA program (n = 31) or one in development (n = 14) (Table 1). Approximately 43% of those with active AYA programs (n = 13) care for > 100 AYAs each year. The majority of active AYA programs (n = 26, 87%) cared for patients across all cancer diagnoses as opposed to being focused on a specific diagnosis. Services offered within active AYA programs varied with almost all providing onco-fertility services (n = 29, 97%) and psychosocial support (n = 28, 93%).
Relationship between pediatric and medical oncology
With regards to geographic proximity, 24% of respondents (n = 14) reported that pediatric and medical oncology providers work in the same building, 48% (n = 28) were located on the same campus and 19% (n = 11) were located on separate campuses. Three quarters of respondents (n = 43) stated they had a single IRB at their institution to approve both pediatric and medical oncology trials. Forty-three percent (n = 25) of respondents stated they had formal joint tumor boards between pediatric and medical oncology for all or some oncologic diagnoses. Almost all participants (n = 52, 90%) reported having ad hoc discussions with their medical oncology colleagues about AYA patients. When asked how often their medical oncology colleagues enrolled their patients onto COG trials, 45% (n = 26) responded that this occurred ‘frequently/occasionally,’ while 41% (n = 24) responded that this ‘rarely/never’ occurred.
Barriers to enrollment
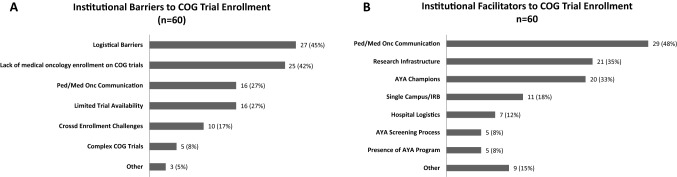
Participants identified several perceived barriers to enrolling AYAs at their local sites with 102 total responses provided by 60 participants (Fig. 1A). The level of agreement on categorization on first review and reconciliation was 86% and 98%, respectively. The remaining 2% of the responses were reconciled by a third reviewer to reach 100% consensus. The most frequently cited barriers to enrolling AYAs included: administrative logistical barriers (45%), perceived lack of interest by medical oncology (42%), communication issues between pediatric and medical oncology (27%) and perceived limited CCT availability (27%). Examples of specific responses categorized as site level barriers are presented in Table 2.
Figure 1.
Reported institutional barriers (A) and facilitators (B) to enrollment of AYA patients onto COG trials.
Table 2.
Reported barriers and facilitators to enrollment.
| Question | Response category | Definition | Example of response |
|---|---|---|---|
| 1. What are the main barriers to accrual of AYA patients onto COG clinical trials at your institution? If applicable, please include barriers to collaboration with medical oncology for clinical trial accrual in your response | Administrative logistical barriers | Administrative barriers at site level that negatively impact AYA trial enrollment | ‘Perceived age barrier by hospital executives’ |
| Perceived medical oncology lack of interest | Perceived lack of enthusiasm to enroll AYA patients to trials; refusal to transfer care of AYA patients to pediatric oncology | ‘Some medical oncologists rather keep patients than refer them if they cannot enroll on trial themselves’ | |
| Cross enrollment challenges | Site level regulatory and structural barriers that hinder AYA patients to be enrolled across cooperative group trials and between medical and pediatric oncology | ‘Adult facility is on different campus’ | |
| Pediatric and medical oncology communication issues | Reported negative relationship between medical and pediatric oncology which does not involve regular communication and negatively impacting site level trial enrollment for AYAs | ‘Lack of an established pathway for knowledge sharing between pediatric and medical oncologists’ | |
| Limited trial availability | AYA focused clinical trials are limited in availability at site level | ‘Adult sites and physicians in Australia not able to participate in COG trials’ | |
| Complexity of COG trials | COG trials are deemed to be too burdensome and complicated at institution by members | ‘Perceived complication of COG trials from the medical oncologist point of view – they are often felt to be too complicated and require too many resources to administer in the medical oncology setting’ | |
| 2. What are the main facilitators to accrual of AYA patients (ages 15–39) onto COG clinical trials at your institution? If applicable, please include facilitators to collaboration with medical oncology for clinical trial accrual in your response | AYA champions | Existence of an individual at institution with focus on AYA clinical trial enrollment at site level | ‘We have a champion within the medical oncology group who is able to enroll patients on COG trials’ |
| Supportive research infrastructure | Presence of a research infrastructure deemed conducive to AYA clinical trial enrollment at site level | ‘Strong clinical research infrastructure at my institution allows us to have most non-phase 1 studies open.’ | |
| Good pediatric and medical oncology communication | Reported positive relationship between medical and pediatric oncology involving regular communication positively impacting AYA clinical trial enrollment | ‘Dialogue between adult and peds to triage specially to ensure they have availability to open COG clinical trials has been a facilitator’ | |
| AYA screening process | Processes in place at site level that allows patients to be identified as AYAs and screened for available clinical trials at institution | ‘Our pediatric Clinical Research Group (GRG) CRAs now screen new patient notifications for potential clinical trial eligibility and maintain a database of patients who are screened’ | |
| Hospital logistics | Administrative policies supporting and allowing AYA Clinical trial enrollment | ‘We allow patients to be treated up to age 39 at our Children’s Hospital’ | |
| Presence of formal AYA program | Existence of a dedicated team of individuals at institution providing care to AYA patients | ‘AYA program in place with a co-directorship-pediatric oncologist and a medical oncologist.’ | |
| Single campus/IRB | Institutional structured such that there is one campus and single IRB between medical and pediatric oncology | ‘Singular IRB and CTSR program allowing more providers to be co investigators on trials’ |
Facilitators to enrollment
Participants identified several perceived facilitators to enrolling AYAs in CCTs with 107 total responses provided by 60 respondents (Fig. 1B). The level of agreement on categorization on first review and reconciliation was 77% and 96%, respectively. The remaining 4% were reconciled by a third reviewer to reach 100% consensus. The most frequently reported facilitators to enrollment included strong communication between pediatric and medical oncology (48%), a supportive research infrastructure (35%) and the presence of AYA champions (33%). Specific responses categorized as site level facilitators are presented in Table 2.
Demographic factors associated with perceived barriers and facilitators to enrollment
We next assessed whether institutional demographics (institution type, presence of a formal AYA program and involvement of medical oncology in COG trial enrollment) were associated with specific perceived barriers and facilitators to enrollment (Supplemental Fig. 1). A strong research infrastructure was more likely to be reported as a facilitator by those institutions if medical and pediatric oncology colleagues were physically in the same building or campus than if they were on a different campus (46.4% vs 0%, p = 0.03). No other variables were significantly associated with differences in perceived barriers and facilitators to enrollment. Similar barriers and facilitators were mostly shared across different types of institutions.
Desired institutional changes and support from the COG AYA RI network
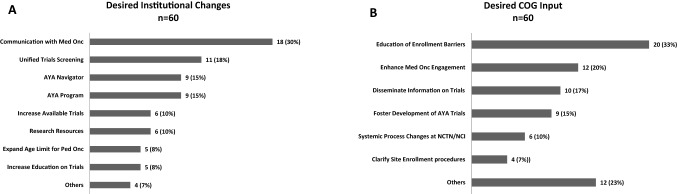
Respondents noted several changes that could potentially improve local enrollment as shown in Fig. 2A. A total of 73 responses from 60 respondents were reviewed and categorized. The level of agreement on categorization on first review and reconciliation was 77% and 95%, respectively. The remaining 5% were reconciled by a third reviewer to reach 100% consensus. The most frequently reported desired changes included improving communication between pediatric and medical oncology (30%), having a unified approach to CCT screening (18%), having an AYA patient navigator (15%) and having an AYA program (15%). Specific examples of these categories are presented in Table 3.
Figure 2.
Reported desired changes at the institutional level (A) and network group level (B) to facilitate and foster AYA clinical trial enrollment.
Table 3.
Recommendations to improve local AYA enrollment.
| Question | Response category | Definition | Example of response |
|---|---|---|---|
| 3. If you could change one thing at your institution to increase accrual of AYA patients to COG clinical trials, what would it be? | Unified trial screening | Processes in place at site level that allows patients to be identified as AYAs and screened for available clinical trials at institution with consensus from medical and pediatric oncology | ‘We will develop a AYA specific program to oversee the screening process and manage patients once identified’ |
| AYA navigator | Existence of an individual at institution with focus on helping AYA patients navigate the clinical trial and treatment experience at site level | ‘We are working to hire a navigator’ | |
| AYA program | Existence of a dedicated team of individuals at institution providing care to AYA patients | ‘Start an AYA clinic with both medical and pediatric oncology’ | |
| Expansion of age limit for pediatric oncology | Institutional policies that allow for non-pediatric patients to be treated by pediatric oncology | ‘Convince my administrators that there is not much difference in treating a 21 vs a 25-year-old’ | |
| Improved communication with medical oncology | Reported positive relationship between medical and pediatric oncology involving regular communication positively impacting AYA clinical trial enrollment | ‘I would really like to simply increase the interaction between Pediatric and medical oncologist’ | |
| Increase in trial availability | Expansion of number of AYA focused trials at site level | ‘More trials available to meet the needs of AYA’ | |
| Research resources | Robust clinical research office and regulatory structure positively impacting AYA trial enrollment | ‘Speed up our IRB!’ | |
| Trial education | Communication and education of medical care providers and community about AYA focused trials and its importance | ‘Increase knowledge in the community regarding our clinical trials’ | |
| 4. How can the AYA COG RI Initiative foster successful accrual of AYA’s to clinical trials at your institution? | Clarify site enrollment procedures | Clear pathways at site level to allow enrollment of AYAs across cooperative groups | ‘Make NCTN cross enrollment workflow clear to all MDs and CRAs’ |
| Enhance medical oncology engagement | Improved communication and relationship with medical oncology partners | ‘Find strategies to reach out to adult oncologists about the benefits of enrollment on peds trials’ | |
| Foster development of AYA trials | Advocating the need to open more AYA focused trials | ‘Helping to voice the need for more available studies’ | |
| Education of enrollment barriers | Exchange of information about barriers to enrollment at specific sites to all AYA RI members | ‘Continued examples of strategies to address common obstacles’ | |
| Disseminate information on trials | Increase knowledge about AYA trials within AYA RI network which leads to more AYAs enrolled on trials | ‘A database could help with being able to identify trials that might be particularly useful for our population’ | |
| Systemic process changes at NCTN/NCI | Initiate NCT/NCI level changes that allow for positive impact on AYA trial enrollment | ‘Making the process of activating COG trials through NCTN as easy as possible’ |
The survey also asked participants to provide recommendations for how the COG AYA RI Network could further support local site’s efforts to increase AYA trial enrollment. A total of 75 responses from 60 respondents were reviewed and categorized. The level of agreement on categorization on first review was 84% and 93% at reconciliation. The remaining 7% were reconciled by a third reviewer to reach 100% consensus. Recommendations included providing education on enrollment barriers (33%), enhancing medical and pediatric oncology engagement (20%), disseminating information on trials (17%), fostering the development of AYA CCTs (15%), fostering process changes at the NCTN/NCI (10%) and clarifying site enrollment procedures (7%) (Fig. 2B). Specific examples are presented in Table 3.
Discussion
In this survey of COG sites with designated AYA RIs, several shared barriers and facilitators to AYA enrollment were identified that appeared to be independent of institutional demographics and infrastructure. The most common shared barriers included poor communication between medical and pediatric oncology, hesitation of cross-enrollment onto cooperative group CCTs, administrative logistical barriers at the institutional level and perception of lack of available CCTs. Leading facilitators to enrollment reported by sites included strong communication between pediatric and medical oncology, supportive research infrastructure and presence of AYA champions.
Apart from logistical barriers, lower cross-enrollment by medical oncologists on COG trials was a major perceived institutional barrier. Limited knowledge of available COG CCTs, lack of time and resources and administrative hurdles (e.g., registering with COG, etc.) may be underlying reasons for poor cross-enrollment by medical oncologists, as documented in previous studies. Importantly, lack of available AYA CCTs is only partially responsible for lower cross-enrollment as some medical oncology programs with similar availability of AYA CCTs also had similar suboptimal accrual rates14–16. Although formal studies are lacking, reports from established AYA programs have hypothesized the benefits of establishing processes that enable medical oncologists and research teams to be aware of available trials and eligible patients17,18. Increasing the number of AYA champions and engaging medical oncologists in disease specific COG CCTs could be potential solutions. Also, where logistically feasible, research coordinators could be shared amongst pediatric and medical oncology provider groups such that familiarity with and knowledge of AYA CCTs is shared between groups.
Perceived lack of availability of AYA CCTs is a shared barrier and has been suggested by multiple prior studies9. Large national efforts, including the reorganization of NCTN which, by consolidating the network groups and supporting closer collaboration, fostered cross network enrollment, have been undertaken and are in process to address this barrier. In our survey, availability of AYA CCTs was often noted to be dependent on the primary oncology service, pediatric or medical oncology. The referral patterns, insurance contracts, administrative policies implementing existing age cut offs for obtaining cancer care largely determine where AYAs get their care and impact their treatment. AYA trials may be more likely to be opened by pediatric oncology sites compared with medical oncology sites19,20 and adolescents treated by adult medical oncologists are less likely to be enrolled in clinical trials16. The hospital treatment setting, community vs academic, also plays a major role. Over 90% of cancer patients under the age of 15 are treated at a tertiary care center versus less than 20% of 15–40-year-old cancer patients21,22. Community oncology practices, where the majority of AYAs are treated, often do not have access to AYA CCTs. Furthermore, there is often limited communication between the pediatric and medical oncologists in the community cancer care setting, and thus, there is often limited knowledge of locally available CCTs21. In addition, the 18-year-old lower age limit of eligibility continues to limit younger AYA participation in CCTs evaluating novel targeted therapies, including immunotherapy trials, and this needs to be further addressed at the national level22.
The current survey further identified potential areas for high-yield interventions to enhance enrollment. Based on this information, key targets for local intervention could include: (1) improving communication between pediatric and medical oncology; (2) employing AYA-specific personnel, such as a patient navigator; and (3) implementing an AYA CCT screening process. Robust communication between pediatric and medical oncology services was perceived as a strong facilitator to CCT enrollment. Increased interaction via tumor boards have been effective in fostering communication between different services and disciplines23. Tumor boards represent an opportunity to review open trials, identify eligible patients and identify optimal treatment approaches for patients with rare cancers and/or complicated presentations. Regular attendance and visibility of AYA team members at shared multidisciplinary tumor boards (MTBs) is crucial to establishing referral networks, enabling ongoing screening of eligible patients and facilitating enrollment24. One example is the EORTC-SPECTA, a virtually conducted molecular profiling MTB specifically focused on recruiting AYA patients with newly diagnosed relapsed high-grade gliomas and high-grade bone and soft tissue sarcomas. A virtual central pathology review with a clinically-validated molecular profiling report is provided to referring clinicians to improve access to novel drugs for AYAs25. Similarly, virtual MTBs may help knowledge sharing across disciplines and improve collaboration between providers and geographically-limited centers as evidenced during the ongoing pandemic26.
AYA programs also foster communication between pediatric and medical oncology. While mostly limited to single institution reports, AYA programs appear to be associated with improved CCT participation due to dedicated staff connecting AYA patients with CCTs27–29. However, the financial implications of developing an AYA program and allocating additional resources to clinical research are barriers. Philanthropic and institutional financial and non-financial support is often needed to launch such initiatives.
Patient navigators and supportive care professionals are well-poised to identify and address needs, values and communication styles of AYA cancer patients and survivors can serve as a conduit for identifying eligible patients for CCTs and relieve some responsibility from the primary medical team. One paramount role of the patient navigator is to serve as a first point of contact for the patient’s care team, with the ability to collaborate with other departments30. In this role, navigators can help bridge the knowledge gap of available AYA CCTs across pediatric and medical oncology departments, as is being studied by the AYA Program in Utah31.
The development of screening procedures to capture eligible AYAs can also enhance AYA CCT enrollment. Implementation of a standard operating procedure for screening at a cancer treatment site improved access and referral to NCTN AYA CCTs presenting to different oncology providers at an academic site. (verbal communication Grimes) Additional studies are needed to identify optimal screening procedures.
A framework for interventions based on the information obtained from the survey has been presented in Table 4. National opportunities to improve AYA enrollments were also highlighted. Maximizing the availability of AYA CCTs for local sites to open has the potential to increase enrollment in so far as the local site is interested in the study question and willing to open the trial. The development of cross-network AYA CCTs was an important step taken by NCTN to improve AYA accrual. While there have been some challenges to cross-enrolling AYAs on cross-network CCTs due to limited knowledge of the cross-enrollment process and differences in treatment approaches between pediatric and medical oncologists, many of these barriers are currently being addressed. The network groups across the NCTN are co-developing concepts at earlier stages of trial design and development and numerous efforts have been made to increase awareness of the cross-enrollment process, including the development of cross-network enrollment frequently asked questions. These efforts have led to a record number of AYA trials available through the NCTN and the number and diversity of these trials is rapidly increasing. Enrollment on these trials is also increasing, as evidenced by the SWOG-led Hodgkin lymphoma trial S1826, in which COG has enrolled more than 30% of the patients to date.
Table 4.
Framework for barriers identified and changes desired and implemented.
| Barriers expressed | Desired institutional change | Ways COG can foster this change | Interventions currently implemented or in process |
|---|---|---|---|
| Poor communication between medical oncology and pediatric oncology |
Improve communication with medical oncology AYA Program with medical oncology AYA Navigator |
Enhance Medical oncology engagement Educate regrading enrollment barriers Disseminate trial information |
Include medical oncologists in AYA RI Network /Joint leadership NCTN cross-network trial development AYA RI network group webinars focusing on barriers and facilitators to enrollment |
| Administrative logistical barriers |
Unified trial screening Expand age limit for pediatric oncology intake AYA Program Shared AYA Navigator |
Foster development of AYA Trials Clarify site enrollment procedures |
NCTN cross-network trial development |
| Cross-enrollment challenges |
Unified trial screening Increased research resources |
Clarify site enrollment procedures Systemic NCTN changes |
Development of COG FAQ NCTN cross-network trial development |
| Complex COG trials | Increase education on trials | Educate and disseminate information on current trials | AYA RI Network webinars focused on individual AYA relevant trials |
| Trials not available | Increase research resources | Foster development of AYA Trials | NCTN cross-network trial development |
While increasing the availability of studies is an important step to increasing enrollment, provider variability in practice and awareness may be more challenging to address at a national level given the diversity of clinical practices and patient populations. However, addressing provider hesitancy and lack of collaboration is critically important. Our survey suggests that education on AYA CCT enrollment processes and trial availability may help to overcome some of the provider hesitancy in offering CCTs to AYAs. The AYA RI Network and NCORP have developed efforts to directly address this gap in knowledge10. Through a series of webinars, these groups have sought to increase interaction amongst AYA oncology providers to disseminate information on available trials and provide guidance on overcoming local barriers to AYA CCT enrollment. Expanding pediatric and medical oncology provider participation in these webinars might be an opportunity to further increase the reach of these educational endeavors.
This study has a few limitations. The RI Network consists of individuals who have an interest in addressing disparities in AYA enrollment and are likely more aware about AYA trials and enrollment processes than their colleagues. Thus participant responses may not be fully representative of other stakeholders, particularly those at sites that are not participating in the AYA RI Network. Further, the RI Network consists mostly of pediatric oncologists, and while the current survey did not assess participants’ role, it is likely that the survey captured a limited number of perspectives from medical oncology stakeholders. It will be extremely important in future studies to obtain a broader perspective from medical oncologists working in varied practice settings on the barriers, enablers, and the feasibility of the proposed strategies to improve accrual. However, stakeholders representing the supportive disciplines and regulatory office were surveyed to capture different perspectives. In addition, the survey focused on medical oncologists cross-enrolling on COG trials and did not include perspectives on pediatric oncologists or medical oncologists enrolling AYA patients on other network group trials. Further studies evaluating medical oncology stakeholder perspectives are needed.
To our knowledge, the extent to which barriers and facilitators to AYA CCT enrollment are shared among institutions has not been previously reported. The shared barriers and facilitators identified in this study provide prime targets for potential intervention to improve enrollment. Studies addressing, and not just describing, the dismal enrollment of AYA CCT enrollment are urgently needed and our survey highlights starting points to begin to optimize the process.
Supplementary Information
Author contributions
N.M., A.S., D.F. and M.R. participated in the development of the study concept, performed data analysis and interpretation of the data, and participated in the writing of the manuscript. V.A. and V.M. participated in the interpretation of the data and writing of the manuscript.
Funding
This work was supported by grants U10-CA180886 (MR, DRF) and P30 CA016672 (MR) from the National Cancer Institute at the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the design of the study, conduct of the study, analysis, interpretation of data, or decision to submit the manuscript for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nupur Mittal and Aniket Saha.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-07703-5.
References
- 1.National Cancer Institute. Adolescents and young adults with cancer. https://www.cancer.gov/types/aya. Accessed 28 Dec 2018.
- 2.Parsons HM, Harlan LC, Seibel NL, et al. Clinical trial participation and time to treatment among adolescents and young adults with cancer: Does age at diagnosis or insurance make a difference? J. Clin. Oncol. 2011;29:4045–4053. doi: 10.1200/JCO.2011.36.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nass SJ, Beaupin LK, Demark-Wahnefried W, et al. Identifying and addressing the needs of adolescents and young adults with cancer: Summary of an Institute of Medicine workshop. Oncologist. 2015;20:186–195. doi: 10.1634/theoncologist.2014-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keegan TH, Ries LA, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122:1009–1016. doi: 10.1002/cncr.29869. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan CP, Nápoles AM, Dohan D, et al. Clinical trial discussion, referral, and recruitment: Physician, patient, and system factors. Cancer Causes Control. 2013;24(5):979–988. doi: 10.1007/s10552-013-0173-5. [DOI] [PubMed] [Google Scholar]
- 6.Felgenhauer J, Hooke MC. Regulatory barriers to clinical trial enrollment of adolescent and young adult oncology patients. Pediatrics. 2014;133(Suppl 3):S119–S122. doi: 10.1542/peds.2014-0122H. [DOI] [PubMed] [Google Scholar]
- 7.Freyer DR, Seibel NL. The clinical trials gap for adolescents and young adults with cancer: Recent progress and conceptual framework for continued research. Curr. Pediatr. Rep. 2015;3:137–145. doi: 10.1007/s40124-015-0075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siembida, E. J., et al.. Barriers and facilitators to adolescent and young adult cancer trial enrollment: NCORP site perspectives. JNCI Cancer Spectrum. 2021. [DOI] [PMC free article] [PubMed]
- 9.Friend BD, Baweja A, Schiller G, et al. Clinical trial enrollment of adolescent and young adult patients with cancer: A systematic review of the literature and proposed solutions. Clin. Oncol. Adolesc. Young Adults. 2017;6:51–59. doi: 10.2147/COAYA.S70375. [DOI] [Google Scholar]
- 10.Siembida EJ, Loomans-Kropp HA, Trivedi N, et al. Systematic review of barriers and facilitators to clinical trial enrollment among adolescents and young adults with cancer: Identifying opportunities for intervention. Cancer. 2020;126(5):949–957. doi: 10.1002/cncr.32675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barakat et al., 2014 JAYAO; Forcina et al., 2018 Adolescent Health, Medicine and Therapeutics.
- 12.Forcina V, Vakeesan B, Paulo C, Mitchell L, Bell JA, Tam S, Wang K, Gupta AA, Lewin J. Perceptions and attitudes toward clinical trials in adolescent and young adults with cancer: A systematic review. Adolesc. Health Med. Ther. 2018;13(9):87–94. doi: 10.2147/AHMT.S163121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roth M, Mittal N, Saha A, et al. The Children's Oncology Group adolescent and young adult responsible investigator network: A new model for addressing site-level factors impacting clinical trial enrollment. J. Adolesc. Young Adult Oncol. 2020;9(4):522–527. doi: 10.1089/jayao.2019.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas SM, Malvar J, Tran H, et al. A prospective, observational cohort study comparing cancer clinical trial availability and enrollment between early adolescents/young adults and children. Cancer. 2018;124:983–990. doi: 10.1002/cncr.31127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins CL, Malvar J, Hamilton AS, et al. Case-linked analysis of clinical trial enrollment among adolescents and young adults at a National Cancer Institute-designated comprehensive cancer center. Cancer. 2015;121:4398–4406. doi: 10.1002/cncr.29669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Downs-Canner S, Shaw PH. A comparison of clinical trial enrollment between adolescent and young adult (AYA) oncology patients treated at affiliated adult and pediatric oncology centers. J. Pediatr. Hematol. Oncol. 2009;31:927–929. doi: 10.1097/MPH.0b013e3181b91180. [DOI] [PubMed] [Google Scholar]
- 17.Shaw PH, Boyiadzis M, Tawbi H, et al. Improved clinical trial enrollment in adolescent and young adult (AYA) oncology patients after the establishment of an AYA oncology program uniting pediatric and medical oncology divisions. Cancer. 2012;118:3614–3617. doi: 10.1002/cncr.26634. [DOI] [PubMed] [Google Scholar]
- 18.Reed DR, Oshrine B, Pratt C, et al. Sink or collaborate: How the immersive model has helped address typical adolescent and young adult barriers at a single institution and kept the adolescent and young adult program afloat. J. Adolesc. Young Adult Oncol. 2017;6(4):503–511. doi: 10.1089/jayao.2017.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Rojas T, Neven A, Terada M, et al. Access to clinical trials for adolescents and young adults with cancer: A meta-research analysis. JNCI Cancer Spectr. 2019;3(4):pkz057. doi: 10.1093/jncics/pkz057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Saghir NS, Keating NL, Carlson RW, et al. Tumor boards: Optimizing the structure and improving efficiency of multidisciplinary management of patients with cancer worldwide. Am. Soc. Clin. Oncol. Educ. Book. 2014 doi: 10.14694/EdBook_AM.2014.34.e461. [DOI] [PubMed] [Google Scholar]
- 21.Yeager ND, Hoshaw-Woodard S, Ruymann FB, et al. Patterns of care among adolescents with malignancy in Ohio. J. Pediatr. Hematol. Oncol. 2006;28:17–22. [PubMed] [Google Scholar]
- 22.Albritton KH, Wiggins CH, Nelson HE, et al. Site of onco-logic specialty care for older adolescents in Utah. J. Clin. Oncol. 2007;25:4616–4621. doi: 10.1200/JCO.2006.08.4103. [DOI] [PubMed] [Google Scholar]
- 23.Roth ME, Unger JM, O'Mara AM, et al. Enrollment of adolescents and young adults onto SWOG cancer research network clinical trials: A comparative analysis by treatment site and era. Cancer Med. 2020;9(6):2146–2152. doi: 10.1002/cam4.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mobley EM, Swami U, Mott S, et al. A retrospective analysis of clinical trial accrual of patients presented in a multidisciplinary tumor board at a tertiary health care center and associated barriers. Oncol. Res. Treat. 2020;43(5):196–203. doi: 10.1159/000506840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Rojas T, Kasper B, Van der Graaf G, et al. EORTC SPECTA-AYA: A unique molecular profiling platform for adolescents and young adults with cancer in Europe. Int. J. Cancer. 2020;147(4):1180–1184. doi: 10.1002/ijc.32651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dharmarajan H, Anderson JL, Kim S, et al. Transition to a virtual multidisciplinary tumor board during the COVID-19 pandemic: University of Pittsburgh experience. Head Neck. 2020;42(6):1310–1316. doi: 10.1002/hed.26195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanford SD, Beaumont JL, Snyder MA, et al. Clinical research participation among adolescent and young adults at an NCI-designated Comprehensive Cancer Center and affiliated pediatric hospital. Support Care Cancer. 2017;25(5):1579–1586. doi: 10.1007/s00520-016-3558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall CS, Grimes A. Pediatric physician management as a predictor of clinical trial enrollment in adolescent and young adult cancer patients. J. Adolesc. Young Adult Oncol. 2020;9(2):183–189. doi: 10.1089/jayao.2019.0125. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell L, Tam S, Lewin J, et al. Measuring the impact of an adolescent and young adult program on addressing patient care needs. J. Adolesc. Young Adult Oncol. 2018;7(5):612–617. doi: 10.1089/jayao.2018.0015. [DOI] [PubMed] [Google Scholar]
- 30.LaRosa KN, Stern M, Lynn C, et al. Provider perceptions' of a patient navigator for adolescents and young adults with cancer. Support Care Cancer. 2019;27(11):4091–4098. doi: 10.1007/s00520-019-04687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warner EL, Fowler B, Pannier ST, et al. Patient navigation preferences for adolescent and young adult cancer services by distance to treatment location. J. Adolesc. Young Adult Oncol. 2018;7(4):438–444. doi: 10.1089/jayao.2017.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.