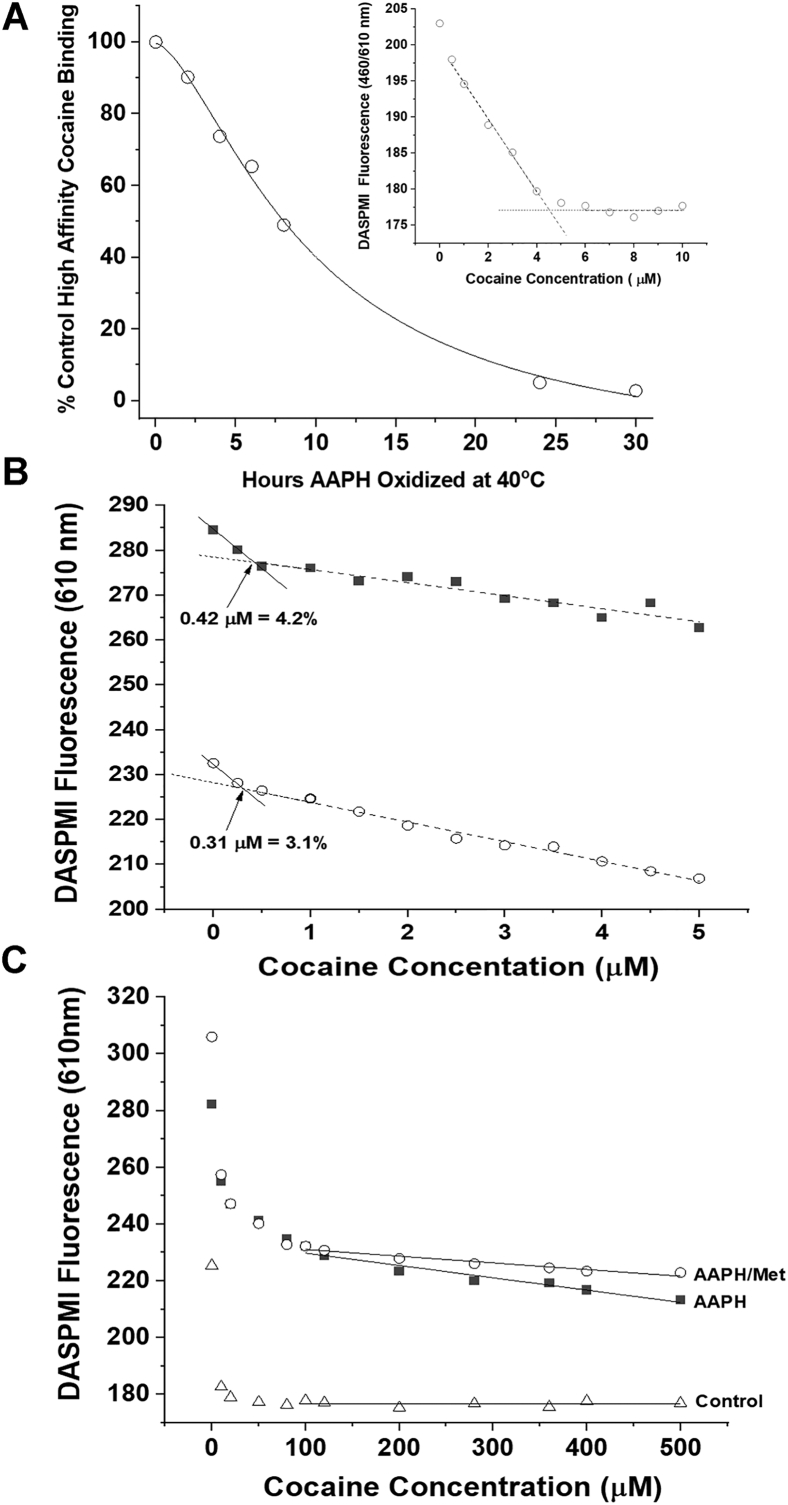
Figure 1.
The effect of AAPH oxidation on cocaine binding to h2E2 mAb.A, the time course of AAPH oxidation induced loss of high-affinity cocaine binding to h2E2 mAb at 40 °C in formulation buffer (plus 260 mM methionine). Inset shows an example of the DASPMI high-affinity cocaine-binding fluorescence assay used to obtain the data, using the control (0 h at 40 °C) mAb as example data. B, DASPMI-binding assay used to determine the percentage of high-affinity cocaine-binding sites remaining, comparing data obtained after 24 h of oxidation at 40 °C with AAPH, in the absence (top) and presence of 260 mM methionine to attenuate methionine oxidation. C, binding of the AAPH-oxidized mAb samples from B titrated to very high concentrations of cocaine, compared with the control h2E2 mAb over the same cocaine concentration range. Straight line fits to data are shown in A (inset), B, and C. Note the nonzero slopes of the straight line fits at the high concentrations of cocaine in the oxidized samples in B and C, indicating that low-affinity cocaine binding is likely present in these oxidized mAb samples. AAPH, 2,2′-azobis(2-amidinopropane) dihydrochloride; DASPMI, 4-(4-(dimethylamino)styryl)-N-methylpyridinium iodide; mAb, monoclonal antibody