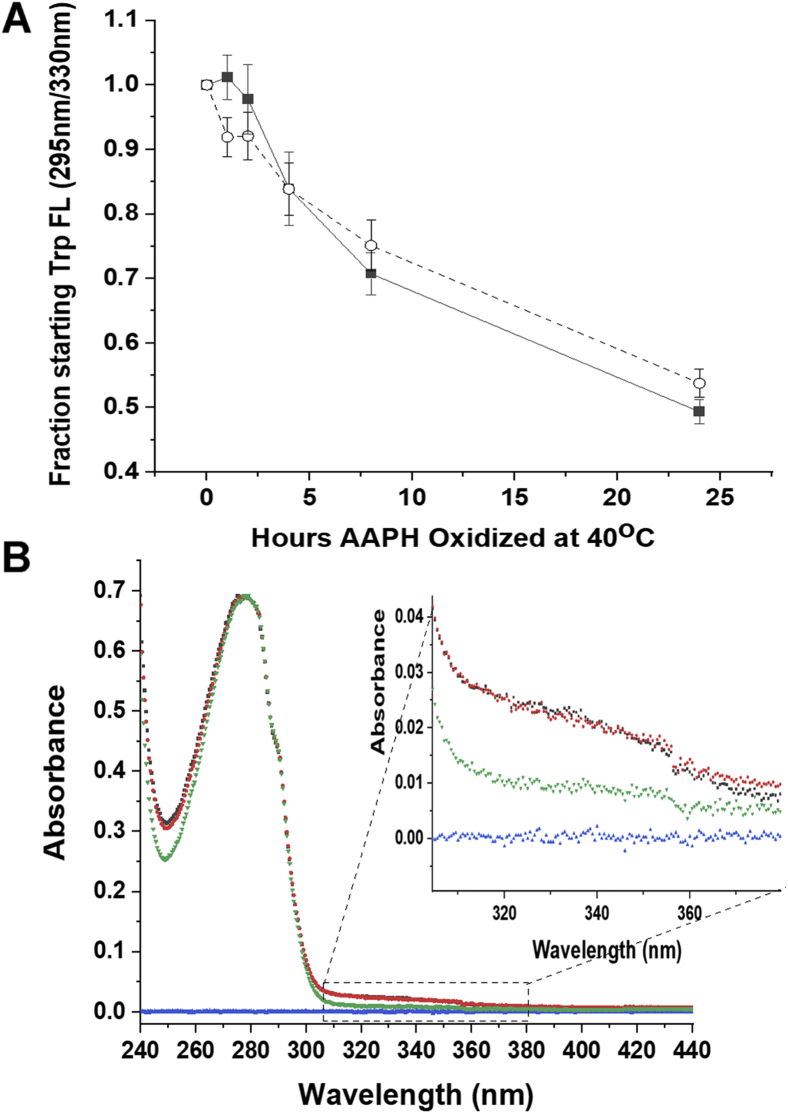
Figure 2.
Spectroscopic effects of AAPH oxidation on the h2E2 mAb.A, the time course of the decrease in tryptophan fluorescence (excitation at 295 nm, emission at 330 nm) upon oxidation with 5 mM AAPH, in the absence (filled squares) and the presence of 260 mM methionine (open circles). Error bars denote the SEMs for three independent experiments. B, the effects of 24 h of 5 mM AAPH oxidation at 40 °C on the absorbance spectrum of the h2E2 mAb. Shown are the buffer blank (blue), untreated mAb (green), AAPH-oxidized mAb (black), and AAPH/260 mM methionine-oxidized mAb (red). The AAPH-oxidized samples were separated from excess reagents using a 40 kDa MWCO spin filter, prior to 40-fold dilution into 20 mM Mops/200 mM NaCl, pH = 7.4 buffer, and spectral measurements. Very little, if any, change in absorbance at 280 nm was observed, so the mAb spectra were normalized to yield the same absorbance at 280 nm for easier visual comparison of spectra. Note the increase in absorbance at 310 to 370 nm (see inset) as well as the increase in the absorbance minimum at about 250 nm because of AAPH oxidation. AAPH, 2,2′-azobis(2-amidinopropane) dihydrochloride; mAb, monoclonal antibody; MWCO, molecular weight cutoff.