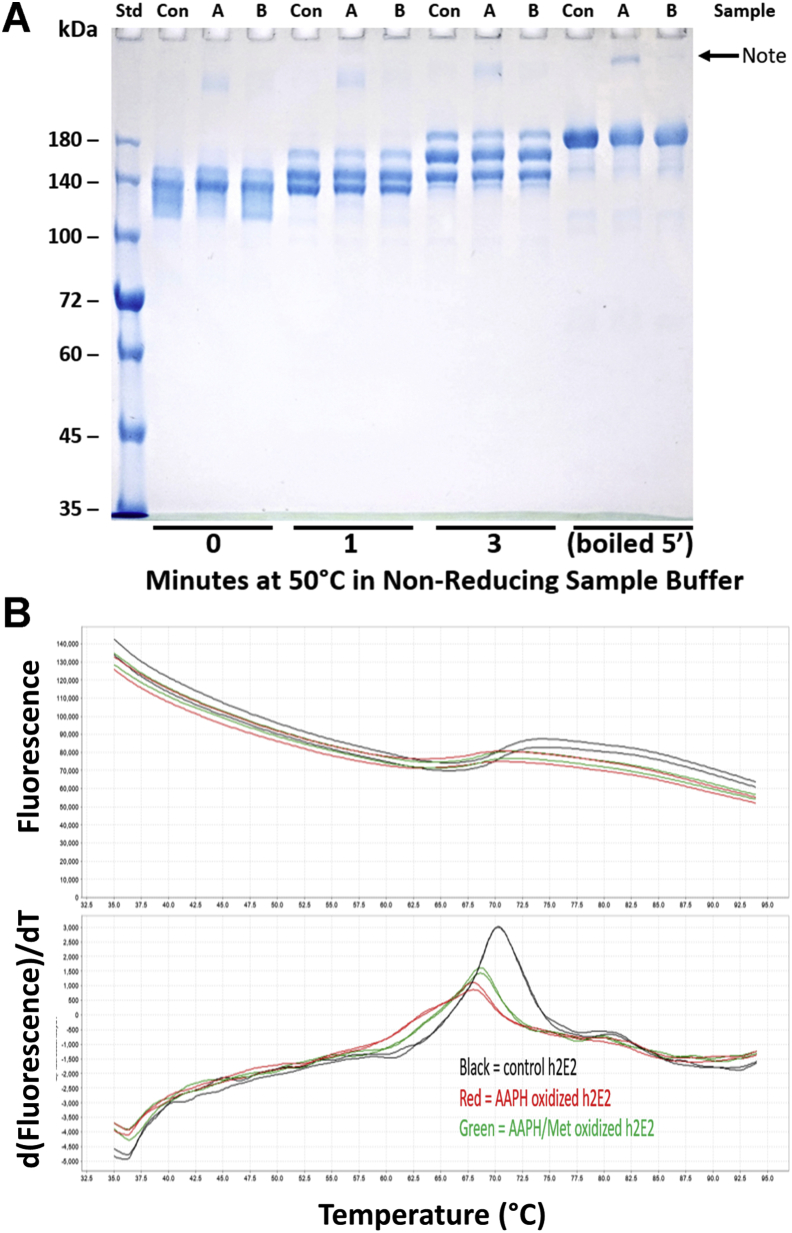
Figure 3.
Comparison of the susceptibility to SDS and thermally induced denaturation for control and AAPH-oxidized mAb.A, time course of thermally induced domain unfolding at 50 °C for untreated (Con), 5 mM AAPH-oxidized (“A”), and 5 mM AAPH/260 mM Met-oxidized mAb (oxidation at 40 °C for 24 h) in a 5% SDS containing Laemmli sample buffer, run on a nonreducing Laemmli gel. Aliquots of these samples were also boiled to completely denature the mAbs (last three lanes). Note that although no obvious differential susceptibility to SDS-induced domain unfolding because of AAPH oxidation is observed, there is a small amount of large molecular weight aggregate evident in the AAPH-treated mAb, which is virtually absent in the AAPH/Met-treated and control mAb. B, the dye fluorescence and first derivative of the dye fluorescence changes observed using differential scanning fluorimetry (DSF) employing 50 μM DASPMI as the reporter dye in PBS buffer. As evident from the decreased temperatures for the derivative plot peak maximums, the oxidized mAb samples (treated for 24 h at 40 °C with 5 mM AAPH, AAPH-mAb in red, and AAPH/Met-mAb in green) are less thermally stable than the control mAb (control mAb is shown in black traces, duplicates of all three samples are shown in B). AAPH, 2,2′-azobis(2-amidinopropane) dihydrochloride; DASPMI, 4-(4-(dimethylamino)styryl)-N-methylpyridinium iodide; mAb, monoclonal antibody.