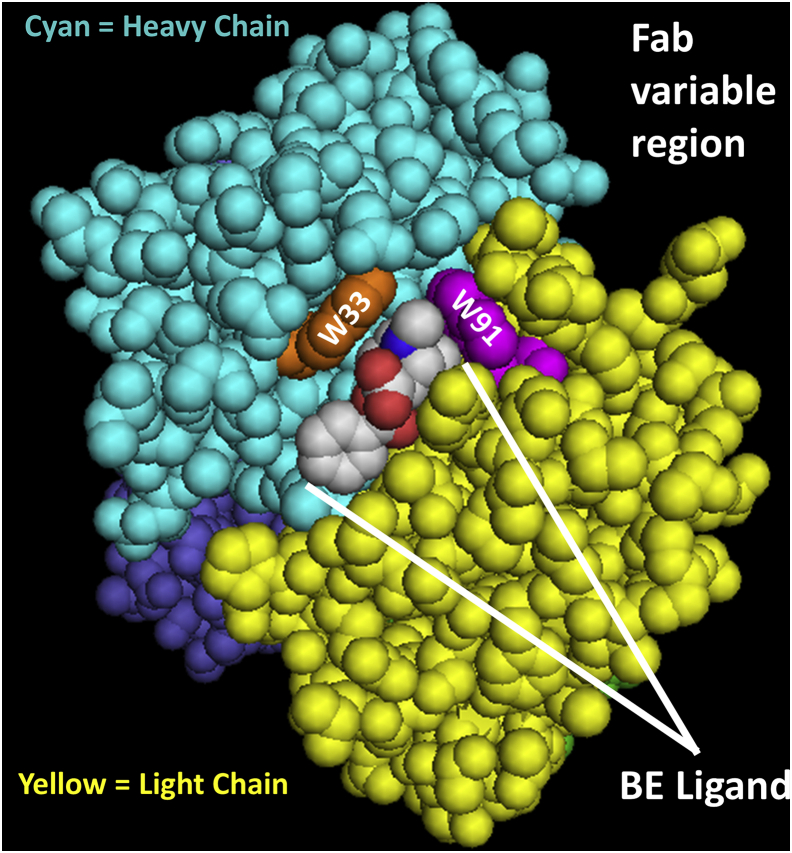
Figure 8.
Crystal structure of the h2E2 mAb Fab fragment (Protein Data Bank code:6NFN) cocrystalized with the benzoylecgonine (BE)cocaine metabolite shown in space filling mode, indicting the location of the light-chain (LC) W91 and the heavy-chain (HC) W33 tryptophan residues shown to be oxidized by AAPH. Only the variable portions of the Fab fragment are shown, with the variable region of the LC in yellow, and the variable portion of the HC in cyan. The BE ligand is shown color coded by element, the LC W91 residue (in the sequence FCALW91YNTHY) is magenta, and the HC W33 residue (in the sequence IFSSDW33M34NW) is orange. Note that these oxidized tryptophan residues are near the tropane ring of the BE ligand, and that this tropane ring is identical in both the cocaine and cocaethylene (CE) structures. The cocaine and CE ligands differ in structure from the BE ligand shown in that the -COOH group on BE, which is directed away from the binding site in this figure, is esterified in cocaine (a methyl ester) and CE (an ethyl ester).