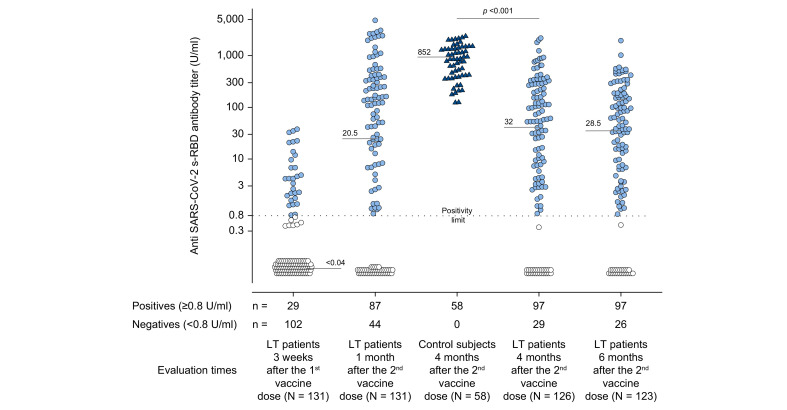
Fig. 2.
Anti-SARS-CoV-2 s-RBD antibody titers evaluated in COVID-19-naïve patients and controls.
In COVID-19-naïve patients, the antibody titers were evaluated 3 weeks (19 days) after the first dose of the Pfizer-BioNTech® BNT162b2 vaccine and after 1 month (31±2 days), 4 months (125±5 days), and 6 months (165±4 days) following the second vaccine dose. Four months (134±15 days) after the second vaccine dose, antibody titers were evaluated in controls. Positive responders to vaccination were defined as those having reached an antibody titer ≥0.8 U/ml (light blue circles for patients and dark blue triangles for controls) while antibody titer <0.8 U/ml identified patient non-responders (white circles). Medians of antibody titers are reported for each time point, and the statistical analysis was performed by means of a non-parametric rank-sum (Mann-Whitney) test.