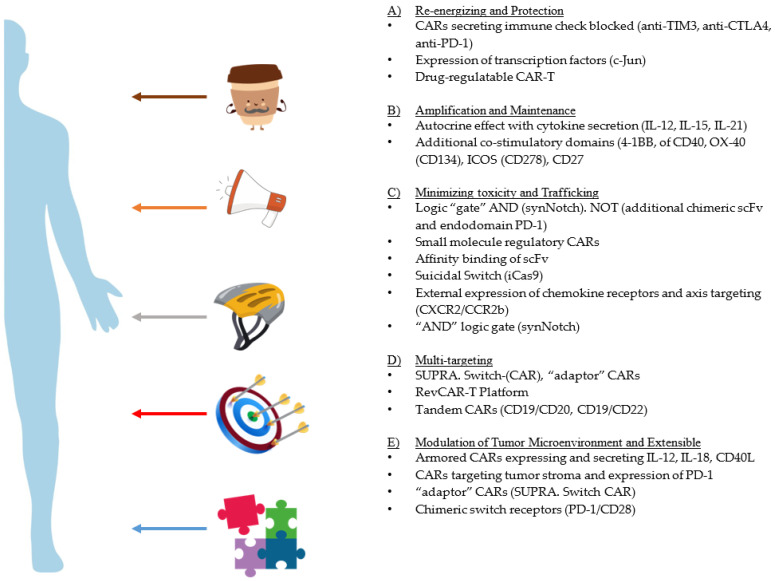
Figure 3.
Preclinical Bioengineering Strategies to Improvise the CARs: An experimental approach with immunocompromised in vivo models is underway to enhance specificity and cognate antigen targeting. These efforts are mitigated to reduce toxicity and increase the validation of the preclinical model. Few of these modifications are under investigation to reverse the exhaustion. (A) Re-energizing of the CAR-T cells has led to the increased persistence as well as reverse resistance to secondary exposure of antigens. Chronic activation of CAR-T cells leads to exhaustive phenotypes. Transient inhibition (“rest”) of CAR signaling has restored the functional phenotype, which was demonstrated by the transcriptional reprogramming and epigenetic remodeling. Enforced ectopic expression of c-Jun and deletion of transcription factor (NR4a) leading to exhaustion have reversed and restored the anti-tumor to an extent. Armored CARs secreting immune checkpoint blocks will be further enhanced by unleashing the breaks on the CAR-T cells. The most important modification identified in all clinical trials consisted of the CAR-mediated cytotoxicity. (B) The combination of CAR secreting IL15 and oncolytic viruses have been recently configured to eliminate tumor cells. Evolution of the third-generation CARs with the integration of multiple co-stimulatory domains (e.g., CD137 (4-1BB)) exhibited better persistence of CARs compared to CD28. The fourth-generation CARs have the ability to constitutively secrete cytokines, such as IL-12, IL-18, IL-21, which have the ability to amplify and expand the CARs as well as autoregulate in a specific environment. (C) To reduce this cytotoxicity, suicide switches (iCasp9), adaptor CARs (RevCARs), and Switch-CARs were engineered. A small molecule and regulatable CARs with an on/off switch were developed to minimize further toxicity. “AND” logic gating, (e.g., synNotch) and bispecific CARs were developed to reduce “on-target off tumor cytotoxicity”. Overexpression of CXCR2/CCR2b axis and CXCR5 were expressed to redirect CAR trafficking to the tumor site and increase mobility to systemic locations. Co-transduction of CARs with various scFvs targeting multiple antigens was designed to reduce cytotoxicity and increase tumor binding specificity. (D) Tandem CAR scFvs (e.g., CD19/CD20, CD19/CD22) were utilized to improve target specificity. Armored CARs secreting IL-12 ad IL-18, as well as CARs targeting the tumor stroma, have recently made an immense difference in the modulation of the tumor microenvironment. (E) Split-CAR design that enables the engineering of multi-feature CAR-T cells, aiming to address current challenges limiting the safety and efficacy of CAR-T cells for cancer treatment (a split, universal, and programmable (SUPRA) CAR).