Figure 1.
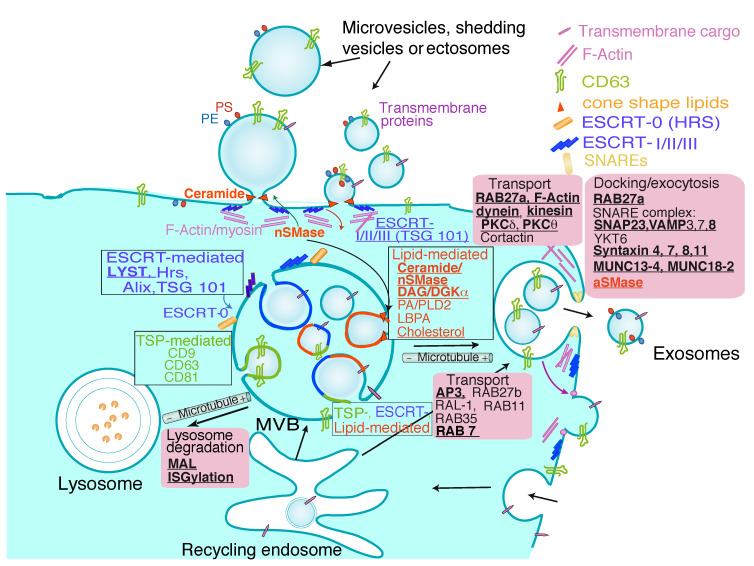
Extracellular vesicles. EV of different intracellular origins can be secreted by eukaryotic cells. The figure represents the different types of vesicles released, either by direct budding from the plasma membrane or by generation of ILV inside MVB, that subsequently fuse with the plasma membrane releasing exosomes. Apoptotic bodies released by dying cells have been excluded. For clarity’s sake, only the constitutive secretion of EV and exosomes is represented, although in certain immune cells such as T and B lymphocytes the traffic of MVB and the secretion of exosomes can be induced via T cell receptor (TCR) and B cell receptor (BCR) stimulation [12]. Traffic of MVB comprises three general phases: ILV biogenesis during the maturation of MVB, transport of MVB to the plasma membrane and docking and fusion of MVB to the plasma membrane, whereas EV secretion involves a single step. Transport and fusion of MVB to the lysosomes may lead to MVB degradation. For more details, please refer to [1,6,13]. General and T lymphocyte-specific mechanisms of shedding vesicles and exosome biogenesis and MVB traffic are represented. The inward, intraluminal budding of specific membrane nanodomains from the MVB limiting membrane produces ILV. The invagination of ILV and the sorting of specific cargoes can be produced by the action of three mechanisms that are enclosed in black line boxes: Endosomal Sorting Complex Required for Transport (ESCRT)-0-I-II-III machinery (blue), tetraspanins (TSP) (green) or certain lipids as cholesterol, ceramide, diacylglycerol (DAG) and lysobisphosphatidic acid (LBPA) (red). In addition, multiple machineries (represented as mixed colors) can collaborate in ILV biogenesis. It is unclear whether the three mechanisms act simultaneously on the same MVB or each one acts on different MVB, although all mechanisms are shown operating in the same MVB for clarity’s sake. Black line rectangles enclose the general mechanisms involved in exosome biogenesis, whereas the regulators of MVB traffic (including transport to lysosomes for degradation, transport to the plasma membrane, docking and fusion with the membrane) are enclosed in magenta boxes. ESCRT-0 components (hepatocyte growth factor-regulated tyrosine kinase substrate -Hrs-, STAM) are generally not observed in plasma membrane budding leading to shedding vesicles, whereas ESCRT-I-II-III are involved in these processes (reviewed in [13]). However, both ESCRT-0 and ESCRT-I-II-III are involved in ILV formation inside MVB [1,13]. Actin cytoskeleton depolymerization is required for secretion of shedding vesicles and exosomes. In addition, externalization of phosphatidylethanolamine (PE) and phosphatidylserine (PS), that binds Annexin V, occurs in plasma membrane-derived EV and, to a lower extent, in exosomes. Bold, underlined characters identify those molecular components or processes that regulate MVB secretory traffic in T lymphocytes: lysosomal trafficking regulator (LYST) [14], neutral sphingomyelinase 2 (nSMase2) [15], DAG [16], diacylglycerol kinase α (DGKα) [17,18,19,20], acidic sphingomyelinase (aSMase) [21], MAL [22,23], ISGylation [24], Adaptor protein 3 (AP3) [25], Rab27a [26], Rab11, Rab7 [27], dynein [28], kinesin-1 [29], cortical F-actin [30,31], centrosomal area F-actin [32,33], protein kinase C δ (PKCδ) [31,34], protein kinase C θ (PKCθ) [35,36], vesicle-associated membrane protein 8 (VAMP-8) [37], syntaxin 4 (STX4) [38], syntaxin 7 (STX7) [39], syntaxin 8 (STX8) [40], syntaxin 11 (STX11) [41], SNAP23 [38]. Underlined characters identify molecules involved in shedding vesicles generation in T lymphocytes: tumor susceptibility gene 101 (TSG101) and vacuolar protein sorting 4 (VPS4) [42]. LYST has Hrs (an ESCRT-associated protein) as binding partner, which supports that LYST participates in MVB biogenesis [43,44].