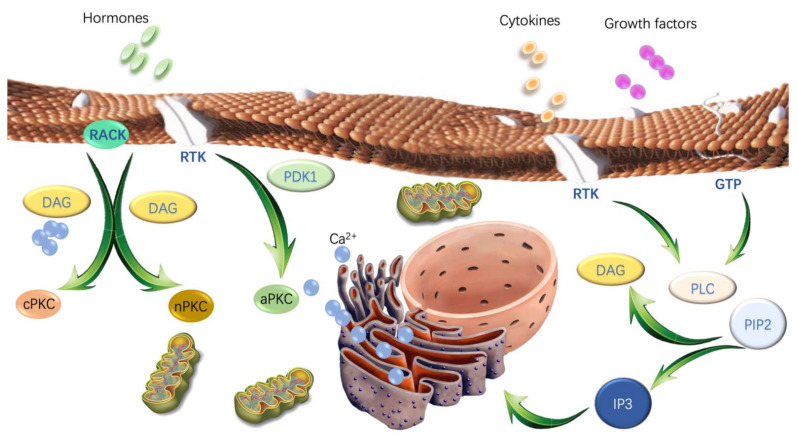
Figure 2.
Schematic representation of the activation of protein kinase C (PKC) isoforms. Before activation, PKC isoforms need to be maturated by 3-phosphoinositide-dependent protein kinase-1 (PDK1). Under physiological conditions, PKC can be activated by interacting with extracellular agonists, such as hormones, cytokines and growth factors, which sequentially translocates to different subcellular compartments, thus conferring specific substrate phosphorylation and distinct cellular functions. The translocation of PKC is regulated by anchoring/scaffolding proteins to ensure proper spatio-temporal distribution of PKC isoforms. Receptors for activated C kinase (RACKs) are intracellular scaffolding proteins that bind to individual PKC isoforms following their activation and provide anchorage of each isoform next to its physiological substrates. Subsequently, the translocated PKC phosphorylates specific substrates, such as membrane-associated phospholipase C (PLC), which hydrolyzes the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) to generate diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). IP3 then interacts with the inositol trisphosphate receptor (InsP3R) and triggers the rapid release of Ca2+ from the intracellular store to the endoplasmic reticulum (ER). DAG is phosphorylated by diacylglycerol kinases (DGK) and converts to phosphatidic acid (PA) in a reaction that terminates PKC-regulated signals.