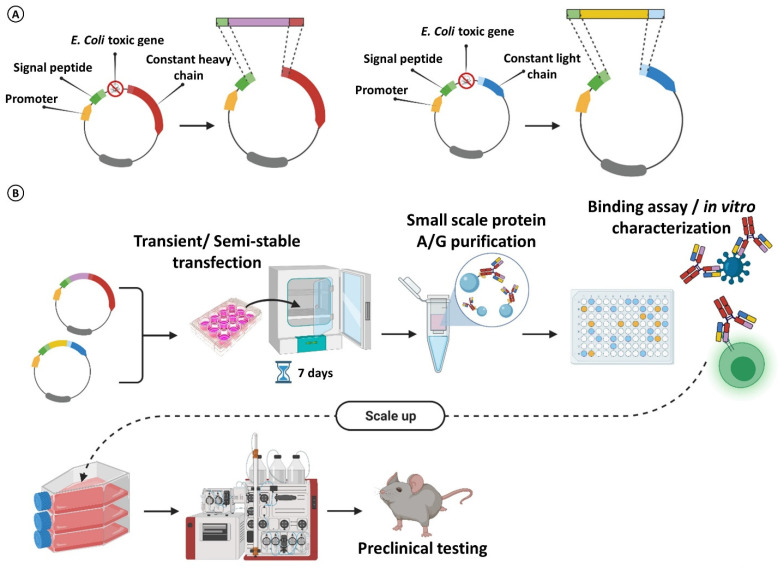
Figure 6.
Workflow for scFvs conversion into full mAbs and small/medium scale production. (A) Variable heavy and light chains of an scFv can be subcloned into mammalian expression vectors encoding constant domains of antibodies. A toxic gene can be used to improve the confidence of cloning. Any format or isotype of interest can be produced (full mAb, scFv-Fc, etc.). (B) Small scale (e.g., 12-well plates) transient/semi-stable transfections in the producer cell lines allow for obtaining enough antibodies for preliminary in vitro testing. The transfection/purification process can be scaled up for preclinical testing.