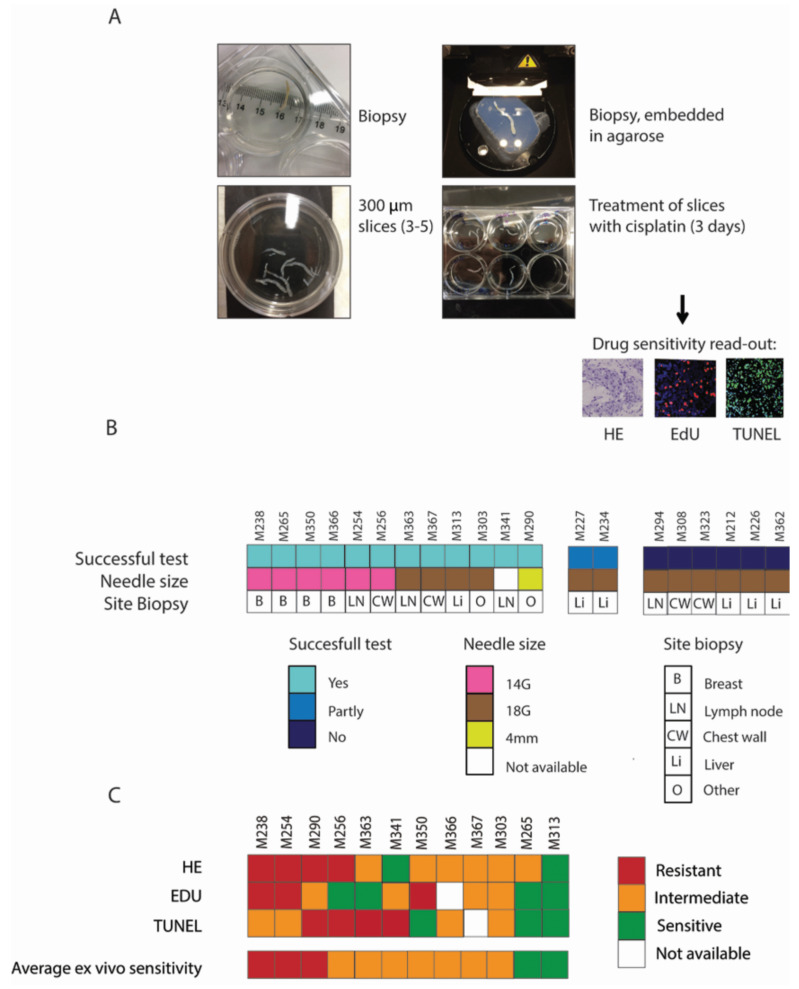
Figure 5.
Feasibility of the ex vivo sensitivity test on organotypic tissue slices from biopsies. (A) Workflow of the ex vivo drug sensitivity screening. Biopsies were embedded in agarose, positioned horizontally, and 300 µM tissue slices were generated. Tissue slices were treated with cisplatin for 3 days and EdU was added 2 hours before fixation. Subsequently, the tissue was formalin fixed and paraffin embedded (FFPE). Drug sensitivity read-out consisted of HE, EdU and TUNEL stainings. (B) Success of the test in relation to the needle size used for the biopsy and the tumor site where the biopsy was taken. A successful test was achieved when sufficient numbers of tumor cells were present in the untreated, 1 μg/mL and 5 μg/mL cisplatin-treated tissue slices. When not all the conditions contained tumor cells, but only the untreated and 1 μg/mL cisplatin condition, the test was considered to be partly successful. When a biopsy contained very little or no tumor cells (n = 4) or was necrotic (n = 2), the test was not successful. (C) Outcome per separate test (HE, EdU and TUNEL) and overall test results, based on the criteria defined in Figure 2A, for all samples with a successful test. M-numbers represent individual mammary tumor biopsies.