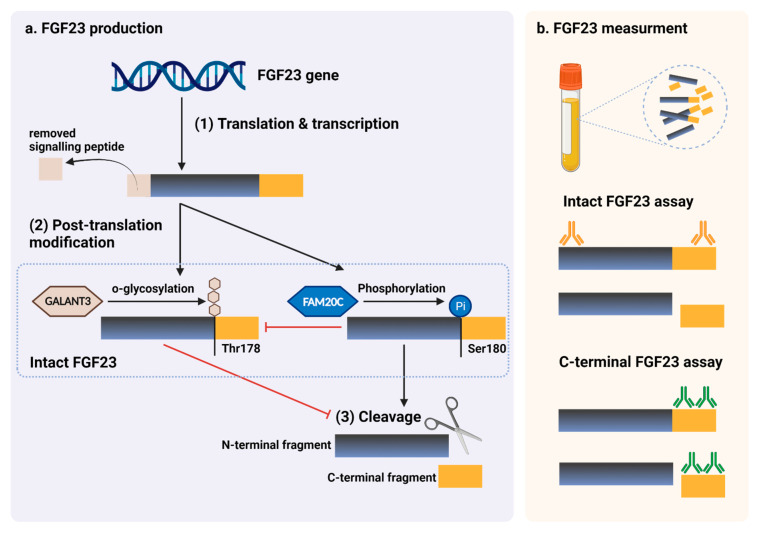
Figure 4.
FGF23 production and immunoassay measurements. (a) After completed transcription and translation, FGF23 can be transferred to two post-translation modification pathways, including O-glycosylation with GALNT3 on Thr178, or phosphorylation by the extracellular serine/threonine protein kinase FAM20C at Ser180. O-glycosylation modification by GALANT3, stabilized form, can prevent intact FGF23 from cleavage. In contrast, phosphorylated FGF23 by FAM20C can be cleaved into N-terminal and C-terminal fragments within the osteocyte/osteoblast. These peptides, including full-length (intact) FGF23, N-terminal fragments, and C-terminal fragments, can be detected in the circulation. (b) For C-terminal assays, detecting antibodies bind to C-terminus epitopes to detect both full-length FGF23 and its C-terminal fragments, whereas assays for intact FGF23 use antibodies to detect epitopes surrounding the FGF23 cleavage site for the detection of only full-length FGF23. This figure was generated with publication licensed by BioRender, Toronto, ON, Canada (Agreement number: DV237SONHF, 19 November 2021). Abbreviations: GALNT3, polypeptide N-acetyl galactosaminyltransferase 3; FAM20C, the extracellular protein kinase FAM20C; Ser, Serine; Thr, Threonine.