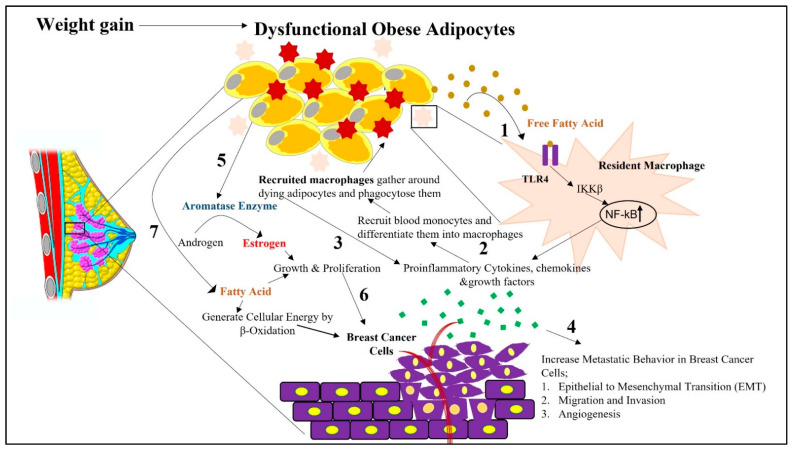
Figure 1.
Schematic representation depicting how obesity-associated changes in white adipocytes adjacent to breast cells in the tumor microenvironment can impact breast cancer initiation and its progression. Impaired physiological function of white adipocytes due to increased fat accumulation, followed by hypoxia and ECM stiffness, causes them to undergo apoptosis-induced cell death. 1. During this cell death phase, cellular contents are released from dying adipocytes. These released contents, such as free fatty acids, cause receptor-mediated activation of TLR4 in resident macrophages. This activation of TLR4 stimulates the secretion of proinflammatory cytokines, chemokines, and growth factors from the resident macrophages via NF-kB activation. 2. From the released chemokines, CCL2 facilitates the recruitment of blood monocytes around the dying adipocytes from the surrounding intravascular spaces. In the presence of environmental cues in the breast tumor microenvironment, these monocytes differentiate into macrophages, which are then considered recruited macrophages. 3. There is further increased intracellular signaling in macrophages via NF-kb-, STAT3-, and JNK-related pathways, followed by the release of proinflammatory cytokines, creating a state of chronic inflammation. 4. Increased secretion of proinflammatory cytokines and hormones from white adipocytes, further facilitates the metastatic progression of breast cancer via their paracrine influence on proximal breast cells. 5. Obesity-associated increased release of proinflammatory cytokines further increases the expression of aromatase in white adipocytes, which then converts androgens to estrogens in adipose tissues. 6. This promotes mammary tumorigenesis by increasing the growth and proliferation of breast cancer cells. 7. Obese adipocyte-released free fatty acids shunt breast cancer cells towards β-oxidation as a source of energy to sustain breast cancer progression.