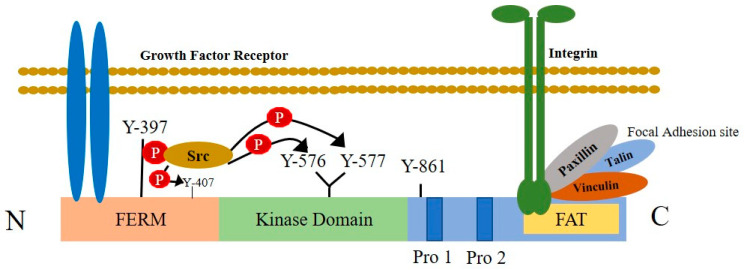
Figure 3.
Structural features of focal adhesion kinase and its activation by cell-surface receptors. FAK is composed of a central kinase domain bordered by the N-terminal FERM homology domain and C-terminal region containing two proline-rich motifs and a FAT domain. The interaction of integrins with extracellular ligands, increases large macromolecular clusters on the cell cytoplasmic side that anchors the actin cytoskeleton to the plasma membrane in connection with the integrin-associated proteins talin, paxillin, and vinculin. This is known as the focal adhesion site. FAK connects with the focal adhesion sites of integrin through its C-terminal domain containing a focal adhesion targeting (FAT) sequence. The N-terminal FERM domain integrates signals from growth factor receptors. In response to extracellular stimuli, FAK activation causes the autophosphorylation of FAK at the Tyr397 residue and creates an SH2 domain docking site that interacts with proteins, such as Src. This interaction activates the Src tyrosine kinase, which further trans-phosphorylates other tyrosine residues placed on FAK and maximizes kinase activity.