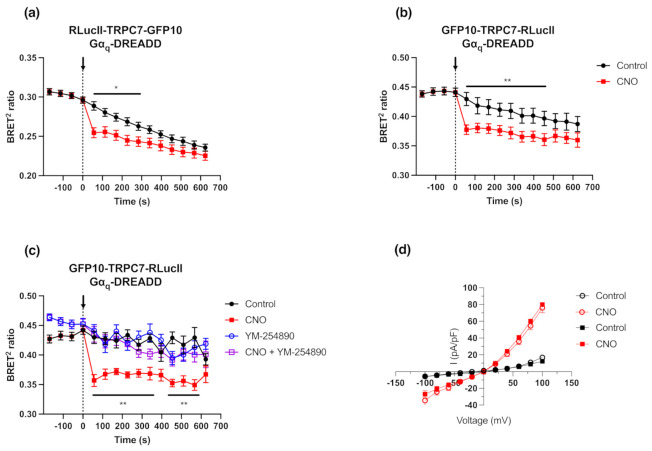
Figure 2.
Double brilliance GFP10-TRPC7-RLucII biosensors allow the detection by BRET of TRPC7 conformational changes in response to Gαq-DREADD activation while being fully functional. (a,b) HEK293 cells were co-transfected with plasmids encoding RLucII-TRPC7-GFP10 or GFP10-TRPC7-RLucII biosensors (75 ng) and Gαq-DREADD (500 ng), and were stimulated with Clozapine-N-Oxide (CNO; 1 µM) or vehicle as a control. The BRET signal was measured for 10 min. (c) HEK293 cells were transfected in the same conditions as in (a) and were pre-incubated with YM-254890 (Gαq inhibitor; 1 µM) or vehicle for 10 min before basal BRET signal was read. The BRET signal was measured for 10 min following stimulation with CNO (1 µM). Each data set represents the mean of three independent experiments, which were each performed in triplicate, and expressed as the mean ± S.E.M. (d) Whole-cell patch-clamp experiments on HEK293 cells were performed 48 h after co-transfection with plasmids encoding Gαq-coupled DREADD and TRPC7 WT (open circles) or the GFP10-TRPC7-RLucII biosensor (closed squares). Current–voltage relationships were obtained from the TRPC7 WT and GFP10-TRPC7-RLucII in control conditions (black symbols) and following Gαq-coupled DREADD stimulation with CNO (red symbols; 1 µM). Each data set represents the mean of seven (7) recordings ± S.E.M. Statistical analyses were performed using a two-way ANOVA with multiple comparisons followed by a Sidak’s post-hoc test. * p < 0.05, ** p < 0.01 for control vs. CNO.