FIGURE 5.
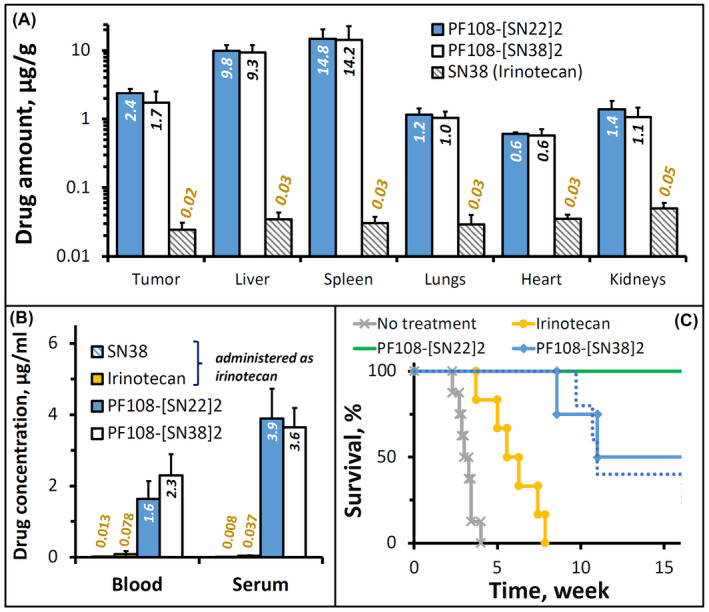
Biodistribution and therapeutic efficacy of PF108‐[SN22]2 in a genetically engineered TH‐MYCN mouse model of de novo resistant, MYCN‐driven NB characterized by overexpression of the ABCG2 transporter. Homozygous 5‐week‐old TH‐MYCN animals with verified large NB tumors were administered PF108‐[SN22]2, PF108‐[SN38]2 or irinotecan intravenously at a dose equivalent to 10 mg/kg of SN22 or SN38. For biodistribution analysis, solid tissue samples (A), blood, and serum (B) were collected 24 h after administering a single dose of the prodrugs or irinotecan (shown as mean ± SD). Alternatively, the compounds were given 2× week for 4 weeks in a therapeutic efficacy experiment (C). The survival of animals administered 1× week for 4 weeks with PF108‐[SN22]2 is shown for comparison as a dotted line
