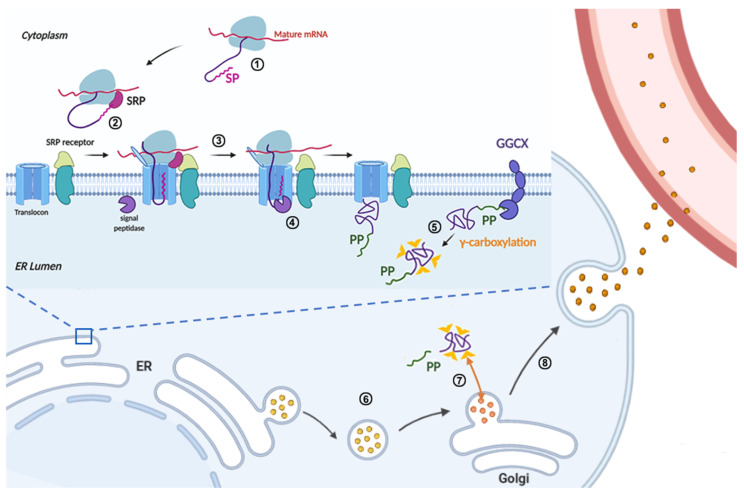
Figure 2.
Schematic diagram of the FIX protein synthesis in liver cells. FIX protein synthesis is initiated in the cytoplasm (①), when the N-terminal signal peptide is synthesized, the signal recognition particle (SRP) recognizes the nascent signal peptide and stops the translation of FIX (②). Directed by SRP, the translation complex is translocated from the cytoplasm to the endoplasmic reticulum (ER) membrane (②), then the ribosome restarts the translation and the nascent polypeptide of FIX is co-translationally translocated into the ER lumen of liver cells (③). At the same time, the signal peptide is cleaved by signal peptidase in the ER lumen side (④). In the ER lumen, the propeptide of FIX directs the γ-carboxylation of glutamate residues in the Gla domain of FIX by an ER integral membrane protein, γ-glutamyl carboxylase (GGCX) (⑤). The fully γ-carboxylated FIX is transported to the Golgi apparatus by membrane coated vesicles (⑥), then the propeptide is cleaved by propeptidase (PACE/Furin) to generate mature FIX in the Golgi apparatus (⑦). The mature FIX is secreted into the blood (⑧). During FIX processing in the ER and Golgi apparatus, multiple post-translational modifications are added, such as N-linked glycosylation, O-linked glycosylation, and β-hydroxylation.