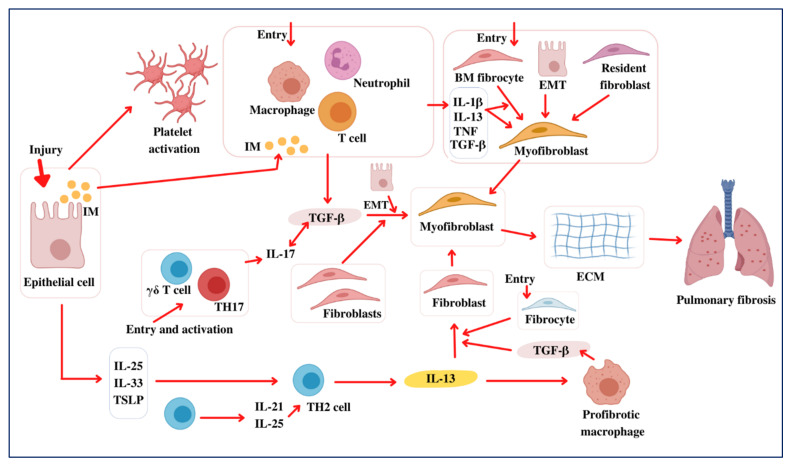
Figure 1.
Pathogenesis of pulmonary fibrosis. Following lung injury, epithelial cells unleash inflammatory mediators (IMs) that activate an antifibrinolytic coagulation mechanism, resulting in platelet stimulation and blood clot initiation. The activated leukocytes secrete profibrotic cytokines, including TNF, IL-1β, IL-13, and TGF-β. Additionally, this enables neutrophils and macrophages to expel dead cells and any aggressive organisms. Subsequently, bone marrow (BM) fibrocytes and resident fibroblasts propagate and develop into myofibroblasts and secrete ECM elements. Furthermore, fibroblasts and myofibroblasts may be produced from epithelial cells that have undergone EMT. The myofibroblasts further facilitate wound healing during the ultimate redecorating and resolution process, resulting in wound contraction and blood vessel regeneration. Fibrosis often occurs if some phase of the tissue regeneration process is poorly developed or if the lung-damaging stimuli continue. TGF-β also acts on epithelial cells, causing EMT and the development of the myofibroblasts that produce ECM. TGF-β1 aggravates the inflammatory activity further by inducing Th17 cell differentiation, leading to PF. Likewise, the epithelial cells produce IL-33, IL-25, and TSLP in response to damage, promoting the production of profibrotic Th2 responses. T cells also have IL-25 and IL-21, which facilitate Th2 differentiation. The Th2 cells produce IL-13, enabling a profibrotic macrophage subpopulation (PMS) that releases TGF-β1 and other mediators. Additionally, unaided IL-13 can actively trigger the fibroblasts of TGF-β1. Consequently, Th2 cytokines induce unique chemokines that facilitate the activity of collagen-secreting fibrocytes (CSFs) from the bone marrow (BM), amplifying the fibrotic responses. As a result, myofibroblasts form to unlock ECM elements and, therefore, lead to PF development.